OROSTAT 8%- epinephrine hydrochloride solution
Orostat 8% by
Drug Labeling and Warnings
Orostat 8% by is a Otc medication manufactured, distributed, or labeled by Gingi-Pak a Division of the Belport, Jeff Nichols. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient
- Purpose
- Description
- Inactive Ingredient
- Keep out of reach if children
- Storage
- Uses
- Do not use
- Warnings
- Directions
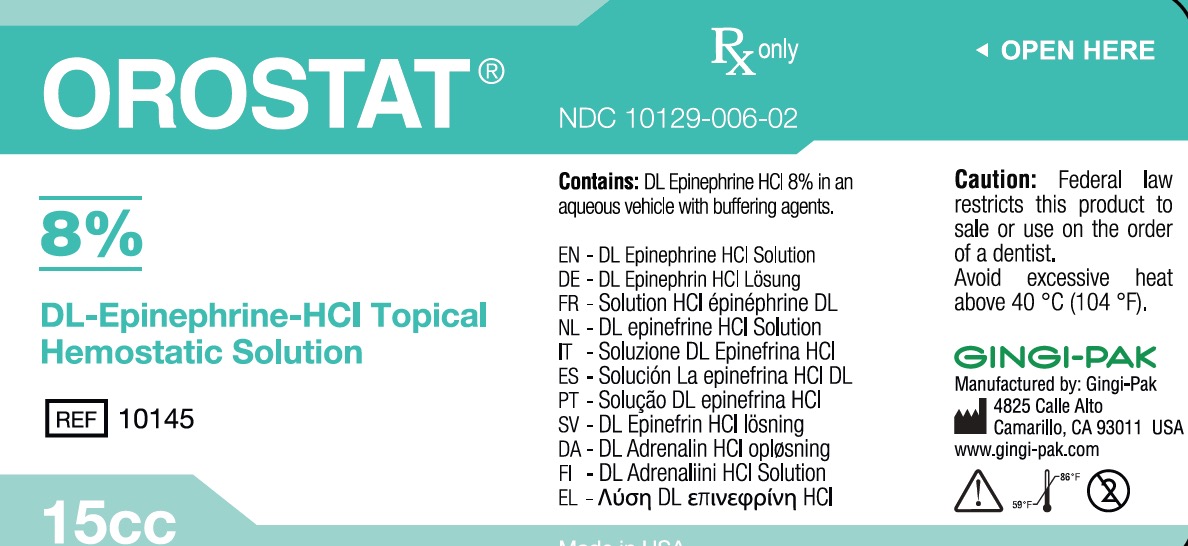
- Principal Display-Orostat 8%
-
INGREDIENTS AND APPEARANCE
OROSTAT 8%
epinephrine hydrochloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 10129-006 Route of Administration SUBGINGIVAL, PERIODONTAL, DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE HYDROCHLORIDE (UNII: WBB047OO38) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE HYDROCHLORIDE 1200 mg in 15 mL Inactive Ingredients Ingredient Name Strength SODIUM DITHIONITE (UNII: 2K5B8F6ES1) 37.5 mg in 15 mL SODIUM THIOSULFATE (UNII: HX1032V43M) 15 mg in 15 mL SODIUM PHOSPHATE, MONOBASIC, ANHYDROUS (UNII: KH7I04HPUU) 37.5 mg in 15 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 49.5 mg in 15 mL PROPYLPARABEN (UNII: Z8IX2SC1OH) 4.95 mg in 15 mL BENZYL ALCOHOL (UNII: LKG8494WBH) 0.15 mL in 15 mL EDETIC ACID (UNII: 9G34HU7RV0) 1.995 mg in 15 mL METHYLPARABEN (UNII: A2I8C7HI9T) 10.05 mg in 15 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10129-006-02 15 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 04/04/1990 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/04/1990 Labeler - Gingi-Pak a Division of the Belport (008480121) Registrant - Jeff Nichols (008480121) Establishment Name Address ID/FEI Business Operations Gingi-Pak a Division of the Belport 008480121 manufacture(10129-006)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.