Contac Cold and Flu Day by Meda Consumer Healthcare Inc. Drug Facts
Contac Cold and Flu Day by
Drug Labeling and Warnings
Contac Cold and Flu Day by is a Otc medication manufactured, distributed, or labeled by Meda Consumer Healthcare Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
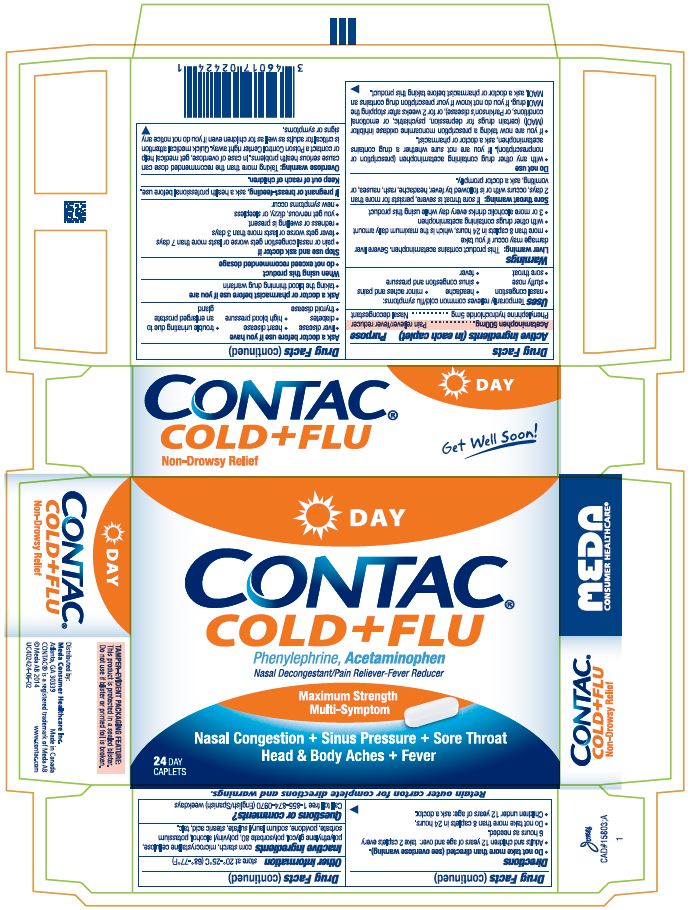
CONTAC COLD AND FLU DAY NIGHT- acetaminophen and phenylephrine hydrochloride tablet
Mylan Consumer Healthcare, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
Temporarily relieves common cold/flu symptoms:
- nasal congestion
- headache
- minor aches and pains
- stuffy nose
- sinus congestion and pressure
- sore throat
- fever
Warnings
-
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
■ more than 8 caplets in 24 hours, which is the maximum daily amount
■ with other drugs containing acetaminophen
■ 3 or more alcoholic drinks every day while using this product
Sore throat warning: If sore throat is severe, persists for more than 2 days, occurs with or is followed by fever, headache, rash, nausea, or vomiting, ask a doctor promptly.
Do not use
-
■ with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
■ if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
-
■ liver disease ■ heart disease ■ trouble urinating due to an enlarged prostate gland
■ diabetes ■ high blood pressure
■ thyroid disease
-
Stop use and ask a doctor if
■ pain or nasal congestion gets worse or last more than 7 days
■ fever gets worse or lasts more than 3 days
■ redness or swelling is present
■ you get nervous, dizzy, or sleepless
■ new symptoms occur
Overdose warning: Taking more than the recommended dose can cause serious health problems. In case of overdose, get medical help or contact a Poison Control Center right away. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
-
■ Do not take more than directed (see overdose warning).
■ Adults and children 12 years of age and over: take 2 caplets every 6 hours as needed.
■ Do not take more than 8 caplets in 24 hours.
■ Children under 12 years of age: ask a doctor.
Inactive ingredients:
corn starch, microcrystalline cellulose, polyethylene glycol, polysorbate 80, polyvinyl alcohol, potassium sorbate, povidone, sodium lauryl sulfate, stearic acid, talc.
Display Panel CONTAC Cold+Flu Day Caplets: 24 count Package
DAY
CONTAC®
COLD+FLU
Phenylephrine, Acetaminophen
Nasal Decongestant/Pain Reliever-Fever Reducer
Maximum Strength
Multi-Symptom
-
Nasal Congestion + Sinus Pressure +Sore Throat
Head & Body Aches + Fever
24 Day Caplets
CONTAC®
COLD+FLU
DAY
Non-Drowsy Relief
Get Well Soon!
Retain outer carton for complete directions and warnings.
TAMPER-EVIDENT PACKAGING FEATURE: This product is protected in a sealed blister. Do not use if blister or printed foil is broken.
-
Distributed by:
Meda Consumer Healthcare Inc.
Atlanta, GA 30339, Made in Canada
CONTAC ® is a registered trademark of Meda AB
© Meda AB 2014 www.contac.com
| CONTAC COLD AND FLU DAY NIGHT
acetaminophen and phenylephrine hydrochloride tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Mylan Consumer Healthcare, Inc. (831410522) |