STERILE CORD BLOOD COLLECTION UNIT (MSC127D)- anticoagulant citrate phosphate dextrose solution injection, solution
Sterile Cord Blood Collection Unit (MSC127D) by
Drug Labeling and Warnings
Sterile Cord Blood Collection Unit (MSC127D) by is a Prescription medication manufactured, distributed, or labeled by Maco Productions, Maco Productions Polonia sp zoo. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
MACO BIOTECH Collection - Sterile Cord Blood Collection Unit (MSC127D)
The MSC127D Sterile Cord Blood Collection Unit consists of 300 mL collection bag containing 27 mL of Anticoagulant Citrate Phosphate Dextrose Solution USP (CPD), a 40 mL rinsing bag containing 8 mL of CPD and two 12 gauge needles with a protective shield (SECUVAM) for the used needle.
Sterile, non-pyrogenic fluid path. Sterilized by steam.
Rx only.
This product is not made with natural rubber latex.
- INDICATIONS AND USAGE
- WARNINGS
-
PRECAUTIONS
- DO NOT use if the overwraps or bags show any signs of alteration.
- This is a single use sterile kit: under no circumstances should it be reused.
- For optimal cord blood quality it is recommended to maintain the cord blood at an ambient temperature (18-26°C / 64.4-78.8°F) or cold temperature (4-12°C / 39.2-53.6°F) and process within 48 hours of collection.1,2,3
If the collection volume is less than 60 mL, the cord blood should be processed withing 24 hours of collection.
- HOW SUPPLIED
-
INSTRUCTIONS FOR USE
Carefully Read All the Instructions Before Use
Representative Product Drawing
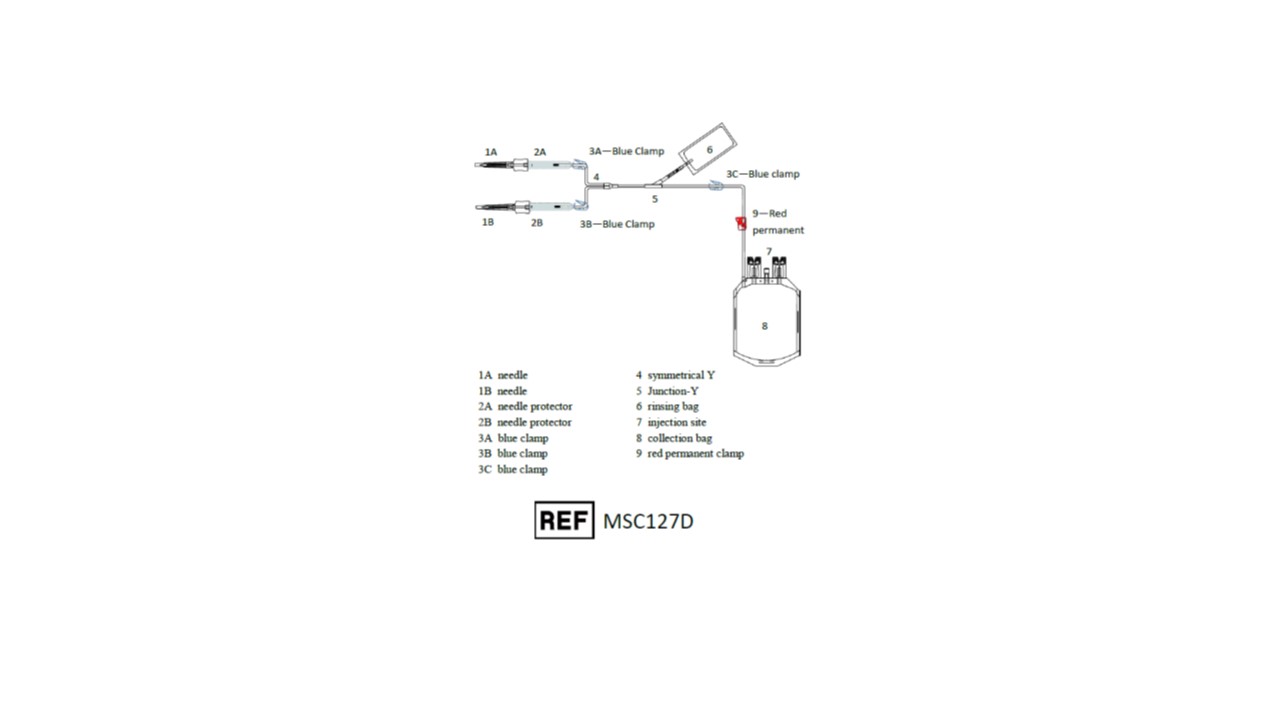
I. PREPARATION- Before use, verify the integrity of the collection unit (overwrap, outlet ports, tubing...).
- Only contents inside the inner wrap are sterile and acceptable for use in a sterile field if pouch is unopened and undamaged.
II. COLLECTION PROCEDURE
- Close all clamps 3A, 3B and 3C (clamp 9 should remain open).
- After clamping and sectioning of the umbilical cord, place the collection bag on a mixer and perform a venipuncture at lower end of the umbilical cord with first needle 1A.
Note: Umbilical venipunctures can be done before or after the expulsion of the placenta according to your local procedures.
3. Open clamps 3A and 3C.
4. Stimulate cord blood flow by massaging the cord from the top downward to increase volume of collection.
5. When collection is completed, close clamps 3A and 3C.
6. Remove needle 1A and slide down SECUVAM 2A (needle protector) over needle 1A.
7. If necessary, perform a second umbilical venipuncture on the upper part of the umbilical cord (near the mother).
[See Warnings]:
a. Open clamps 3B and 3C.
b. Perform venipuncture on the umbilical cord using other needle 1B.
c. When collection is completed, close clamps 3B and 3C.
d. Remove needle 1B and slide down SECUVAM 2B (needle protector) over needle 1B.
III. RINSING OF COLLECTIONLINE
- Open clamp 3C.
- Snap the break-away cannula on top of rinsing bag 6.
- Roll up bag 6 on itself to empty 8 mL of anticoagulant (CPD) in the tubing to push the cord blood remaining in the tubing into the collection bag. Do not strip the tubing.
- Close clamp 3C.
- Proceed to section V END OF COLLECTION if collection volume is less than 200 mL. For collection volumes greater than 200 mL, proceed to section IV ADDITION OF ANTICOAGULANT INTO THE COLLECTION BAG.
IV. ADDITION OF ANTICOAGULANT INTO THE COLLECTION BAG
Use this step only for collection volumes greater than 200 mL.
- Seal the tubing above the Y junction 5.
- Move clamp 3C near to Y junction 5.
- Using a blood stripper, strip the tubing from Y junction 5 towards the collection bag 8 expelling the anticoagulant in the tubing into collection bag 8.
- Repeat this operation 3 times.
- Mix collection bag 8.
- Close clamp 3C.
- Proceed to section V END OF COLLECTION.
V. END OF COLLECTION
- Close clamp 9 to secure th collection.
- Seal the tubing between clamp 9 and collection bag 8.
- Fill in collection volume and CPD volume on the collection bag label. The amount of CPD is as follows:
a. 27 mL - if no additional CPD is added.
b. 35 mL - if additional 8 mL CPD from rinsing bag is added to the collection bag as directed in section IV.
4. For optimal cord blood quality, maintain the cord blood at an ambient temperature (18-26°C / 64.4-78.8°F) or cold temperature (4-12°C / 39.2-53.6°F).
VI. CONTACT INFORMATION
Manufactured for United States of America (U.S.A.) and Canada by Maco Productions Polonia, Sp. z o.o.
ul. Szwajcarska 22, 54-405 Wroclaw, POLAND
+48 71 37 60 110
For consumer information contact:
- For the U.S.A.: Distributed by Uneklo USA, Inc. dba Macopharma USA
3075 Breckinridge Boulevard, Suite 405, Duluth, GA 30096, USA
(770) 270-6867
- For Canada: Distributed by Macopharma Canada
1200 McGill College, Suite 1715, Montreal (Quebec) H3B 4G7
(514) 866-2222
VII. REFERENCES- Pope, B. Mitsakos, K., Bilgin, A., Hokin, B. and Grant, R.: Predicting overall viability of cord blood harvests. Transfusion 2012:52:1079-1085
- Salge-Bartels U. Huber. M, Kleiner K, Volkers P, Seitz R, Heiden M: Evaluation of Quality Parameters for Cord Blood Donations, Transfus Med Hemother 2009; 36:317-324
- Prof. Dr. Kogler, Jose Carreras CBB, medical center, University of Duesseldorf: General information on the institution and the production facility of the Jose Carreras Cord Blood Bank. 2014
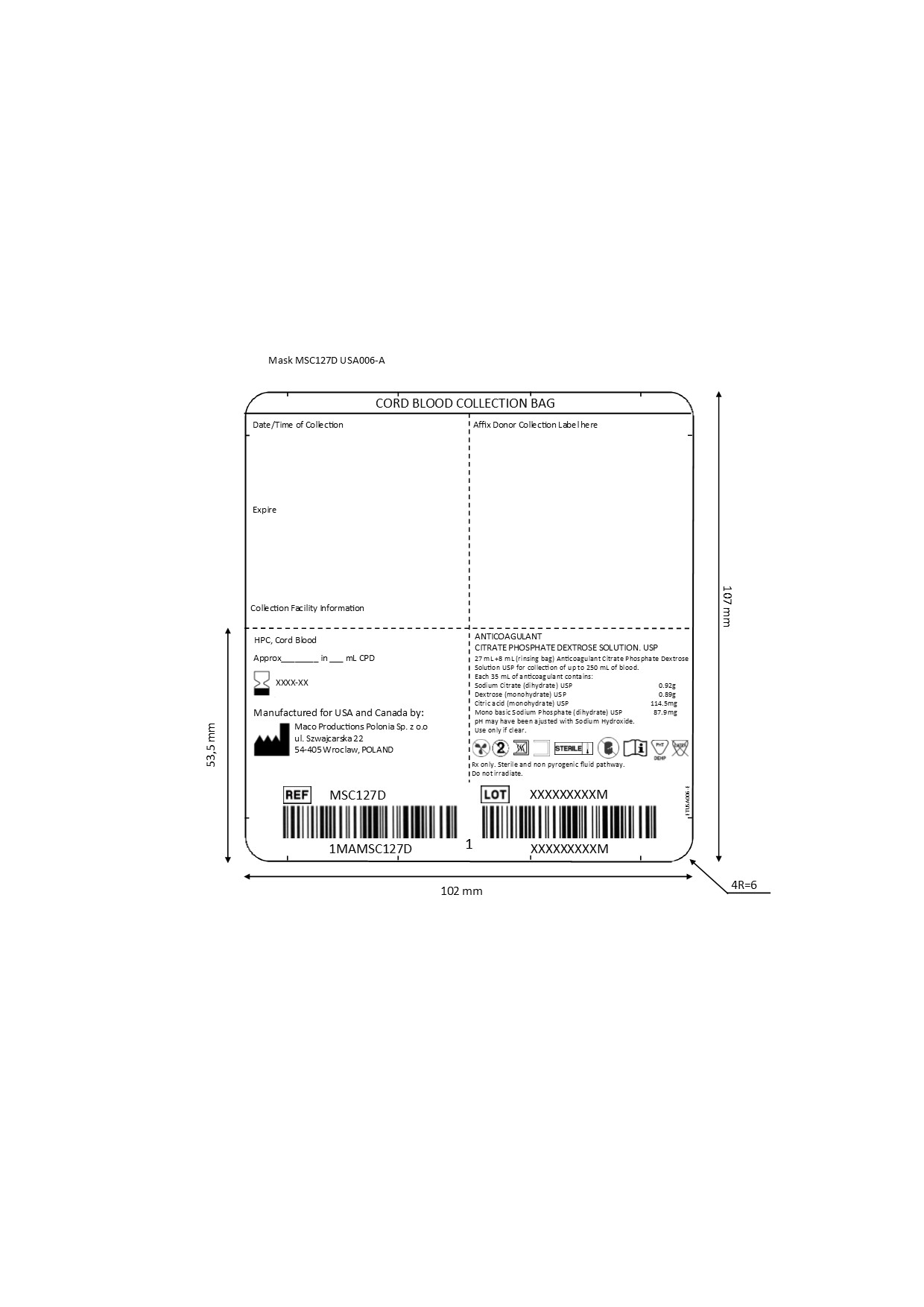
- LABELS
-
INGREDIENTS AND APPEARANCE
STERILE CORD BLOOD COLLECTION UNIT (MSC127D)
anticoagulant citrate phosphate dextrose solution injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 14498-003 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CITRATE (UNII: 1Q73Q2JULR) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) SODIUM CITRATE 0.92 g in 35 mL DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 0.89 g in 35 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 114.5 mg in 35 mL SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) (PHOSPHATE ION - UNII:NK08V8K8HR) SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE 87.9 mg in 35 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 14498-003-01 1 in 1 KIT 1 35 mL in 1 KIT; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN125552 12/21/2016 Labeler - Maco Productions (265492868) Registrant - Maco Productions (265492868) Establishment Name Address ID/FEI Business Operations Maco Productions Polonia sp zoo 367387284 manufacture(14498-003)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.