BABYGANICS KIDS ALCOHOL FREE FOAMING HAND SANITIZER BERRY BERRY- benzalkonium chloride liquid BABYGANICS KIDS ALCOHOL FREE FOAMING HAND SANITIZER CUPCAKE- benzalkonium chloride liquid
BabyGanics Kids Alcohol Free Foaming Hand Sanitizer Cupcake by
Drug Labeling and Warnings
BabyGanics Kids Alcohol Free Foaming Hand Sanitizer Cupcake by is a Otc medication manufactured, distributed, or labeled by KAS Direct LLC dba BabyGanics, Voyant Beauty, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
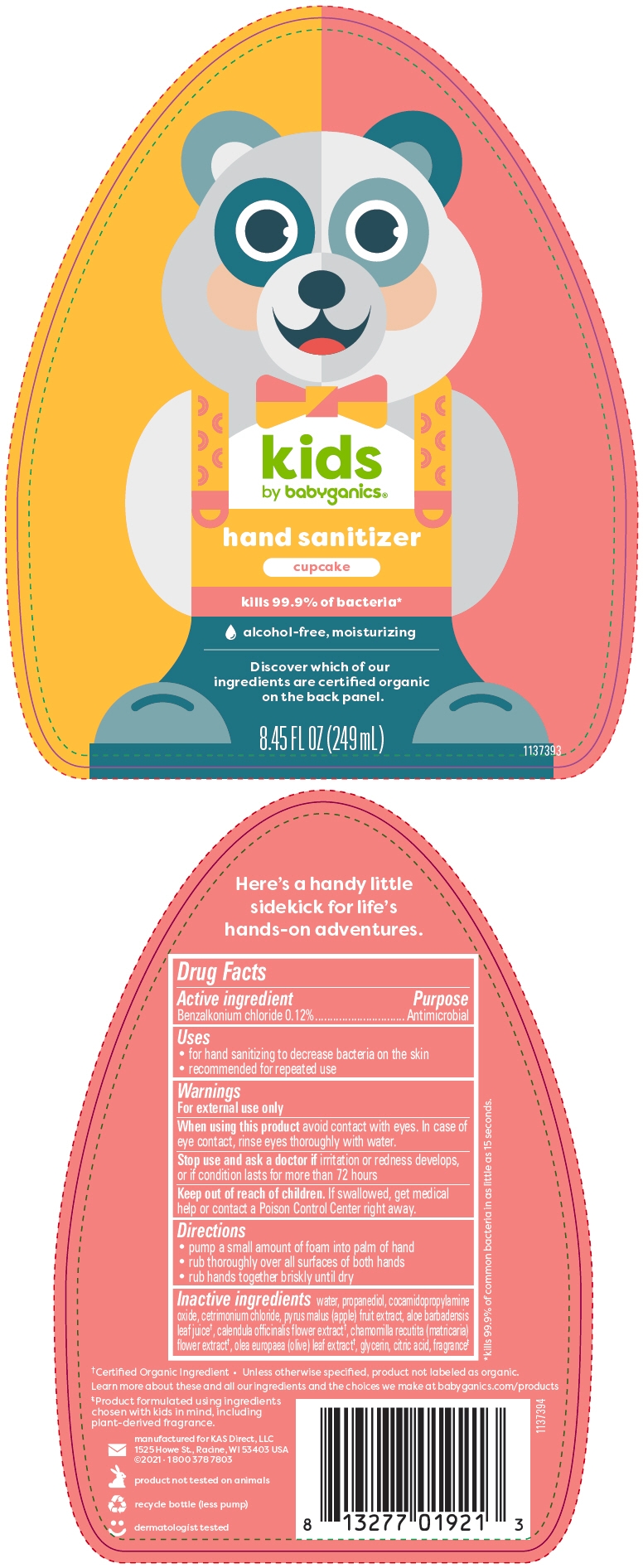
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
-
Inactive ingredients
water, propanediol, cocamidopropylamine oxide, cetrimonium chloride, pyrus malus (apple) fruit extract, aloe barbadensis leaf juice1, calendula officinalis flower extract1, chamomilla recutita (matricaria) flower extract1, olea europaea (olive) leaf extract1, glycerin, citric acid, fragrance2
- 1 Certified Organic Ingredient Unless otherwise specified, product not labeled as organic.
Learn more about these and all our ingredients and the choices we make at babyganics.com/products- 2 Product formulated using ingredients chosen with kids in mind, including plant-derived fragrance.
- 1 Certified Organic Ingredient Unless otherwise specified, product not labeled as organic.
- PRINCIPAL DISPLAY PANEL - 249 mL Bottle Label - NDC: 59062-4101
- PRINCIPAL DISPLAY PANEL - 249 mL Bottle Label - NDC: 59062-4201
-
INGREDIENTS AND APPEARANCE
BABYGANICS KIDS ALCOHOL FREE FOAMING HAND SANITIZER BERRY BERRY
benzalkonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 59062-4101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.12 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPANEDIOL (UNII: 5965N8W85T) COCAMIDOPROPYLAMINE OXIDE (UNII: M4SL82J7HK) APPLE (UNII: B423VGH5S9) ALOE VERA LEAF (UNII: ZY81Z83H0X) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) CHAMOMILE (UNII: FGL3685T2X) OLIVE OIL (UNII: 6UYK2W1W1E) GLYCERIN (UNII: PDC6A3C0OX) CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59062-4101-1 250 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 06/06/2022 12/31/2027 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug 505G(a)(3) 06/06/2022 12/31/2027 BABYGANICS KIDS ALCOHOL FREE FOAMING HAND SANITIZER CUPCAKE
benzalkonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 59062-4201 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.12 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPANEDIOL (UNII: 5965N8W85T) COCAMIDOPROPYLAMINE OXIDE (UNII: M4SL82J7HK) APPLE (UNII: B423VGH5S9) ALOE VERA LEAF (UNII: ZY81Z83H0X) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) CHAMOMILE (UNII: FGL3685T2X) OLIVE OIL (UNII: 6UYK2W1W1E) GLYCERIN (UNII: PDC6A3C0OX) CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59062-4201-1 250 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 06/06/2022 12/31/2027 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug 505G(a)(3) 06/06/2022 12/31/2027 Labeler - KAS Direct LLC dba BabyGanics (002764605) Establishment Name Address ID/FEI Business Operations Voyant Beauty, Inc. 253610042 MANUFACTURE(59062-4101, 59062-4201)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.