TRELSTAR- triptorelin pamoate kit
Trelstar by
Drug Labeling and Warnings
Trelstar by is a Prescription medication manufactured, distributed, or labeled by Allergan, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TRELSTAR safely and effectively. See full prescribing information for TRELSTAR.
TRELSTAR® (triptorelin pamoate for injectable suspension), for intramuscular use
Initial U.S. Approval: 2000
RECENT MAJOR CHANGES
Warnings and Precautions, Embryo-Fetal Toxicity (5.9) 12/2018
INDICATIONS AND USAGE
TRELSTAR is a gonadotropin releasing hormone (GnRH) agonist indicated for the palliative treatment of advanced prostate cancer. (1)
DOSAGE AND ADMINISTRATION
TRELSTAR is administered as a single intramuscular injection in either buttock. Due to different release characteristics, the dosage strengths are not additive and must be selected based upon the desired dosing schedule. (2.1)
DOSAGE FORMS AND STRENGTHS
Injectable suspension: 3.75 mg, 11.25 mg, 22.5 mg. (3)
CONTRAINDICATIONS
- Known hypersensitivity to triptorelin or any other component of the product, or other GnRH agonists or GnRH. (4)
WARNINGS AND PRECAUTIONS
- Hypersensitivity: Anaphylactic shock, hypersensitivity, and angioedema have been reported. In the event of a reaction, discontinue TRELSTAR and initiate appropriate medical management. (5.1)
- Tumor Flare: Transient increase in serum testosterone levels can occur within the first few weeks of treatment. This may worsen prostate cancer and result in spinal cord compression and urinary tract obstruction. Monitor patients at risk and manage as appropriate. (5.2 and 5.3)
- Effect on QT/QTc Interval: Androgen deprivation therapy may prolong the QT interval. Consider risks and benefits. (5.4)
- Hyperglycemia and Diabetes: Hyperglycemia and an increased risk of developing diabetes have been reported in men receiving GnRH analogs. Monitor blood glucose level and manage according to current clinical practice. (5.5)
- Cardiovascular Diseases: Increased risk of myocardial infarction, sudden cardiac death and stroke has been reported in men. Monitor for cardiovascular disease and manage according to current clinical practice. (5.6)
ADVERSE REACTIONS
- 3.75 mg: The most common adverse reactions (≥ 5%) during TRELSTAR 3.75 mg therapy included hot flushes, skeletal pain, impotence, and headache. (6.1)
- 11.25 mg: The most common adverse reactions (≥ 5%) during TRELSTAR 11.25 mg therapy included hot flushes, skeletal pain, headache, edema in legs, and leg pain. (6.1)
- 22.5 mg: The most common adverse reactions (≥ 5%) during TRELSTAR 22.5 mg therapy included hot flushes, erectile dysfunction, and testicular atrophy. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Allergan at 1-800-678-1605 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
None. (7)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
2.2 Reconstitution Instructions for TRELSTAR with MIXJECT SYSTEM
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Transient Increase in Serum Testosterone
5.3 Metastatic Vertebral Lesions and Urinary Tract Obstruction
5.4 Effect on QT/QTc Interval
5.5 Hyperglycemia and Diabetes
5.6 Cardiovascular Diseases
5.7 Laboratory Tests
5.8 Laboratory Test Interactions
5.9 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1
INDICATIONS AND USAGE
TRELSTAR is indicated for the palliative treatment of advanced prostate cancer [see Clinical Studies (14)].
-
2
DOSAGE AND ADMINISTRATION
2.1 Dosing Information
TRELSTAR must be administered under the supervision of a physician.
TRELSTAR is administered by a single intramuscular injection in either buttock. Dosing schedule depends on the product strength selected (Table 1). The lyophilized microgranules are to be reconstituted in sterile water. No other diluent should be used.
Table 1. TRELSTAR Recommended Dosing Dosage 3.75 mg 11.25 mg 22.5 mg Recommended dose 1 injection every
4 weeks1 injection every
12 weeks1 injection every
24 weeksDue to different release characteristics, the dosage strengths are not additive and must be selected based upon the desired dosing schedule.
The suspension should be administered immediately after reconstitution.
As with other drugs administered by intramuscular injection, the injection site should be alternated periodically.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
2.2 Reconstitution Instructions for TRELSTAR with MIXJECT SYSTEM
Please read the instructions completely before you begin.
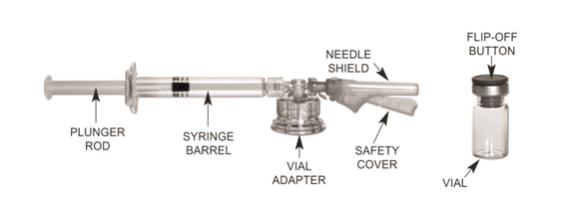
MIXJECT Preparation
Wash your hands with soap and hot water and put on gloves immediately prior to preparing the injection. Place the sealed tray on a clean, flat surface that is covered with a sterile pad or cloth. Peel the cover away from the tray and remove the MIXJECT components and the TRELSTAR vial. Remove the Flip-Off button from the top of the vial, revealing the rubber stopper. Place the vial in a standing upright position on the prepared surface. Disinfect the rubber stopper with the alcohol wipe. Discard the alcohol wipe and allow the stopper to dry. Proceed to MIXJECT Activation.
MIXJECT Activation
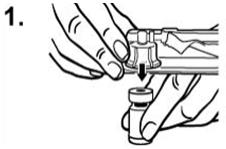
Peel the cover away from the blister pack containing the vial adapter. Do not remove the vial adapter from the blister pack. Place the blister pack containing the vial adapter firmly on the vial top, piercing the vial. Push down gently until you feel it snap in place. Remove the blister pack from the vial adapter. 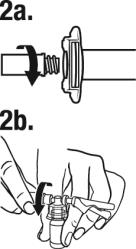
(a) Screw the plunger rod into the barrel end of the syringe. Remove the cap from the syringe barrel.
(b) Connect the syringe to the vial adapter by
screwing it clockwise into the opening on the side of the vial adapter. Be sure to gently twist the syringe until it stops turning to ensure a tight connection.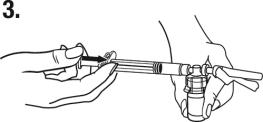
While holding the vial, place your thumb on the plunger rod and push the plunger rod in all the way to transfer the diluent from the pre-filled syringe into the vial. Do not release the plunger rod. 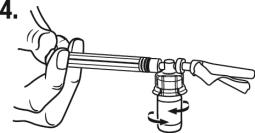
Keeping the plunger rod depressed, gently swirl the vial so that the diluent rinses the sides of the vial. This will ensure complete mixing of TRELSTAR and the sterile water diluent. The suspension will now have a milky appearance. In order to avoid separation of the suspension, proceed to the next steps without delay. 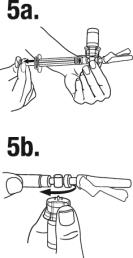
(a) Invert the MIXJECT system so that the vial is at the top.
Grasp the MIXJECT system firmly by the syringe and pull back the plunger rod slowly to draw the reconstituted TRELSTAR into the syringe.
(b) Return the vial to its upright position, and disconnect the vial adapter and vial from the MIXJECT syringe assembly by turning the plastic cap of the vial adapter clockwise.
Grasp only the plastic cap when removing.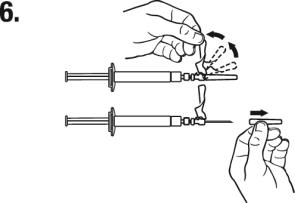
Lift up the safety cover and remove the clear plastic needle shield by pulling it from the assembly. The safety cover should be perpendicular to the needle, with the needle facing away from you. The syringe containing the TRELSTAR suspension is now ready for administration. The suspension should be administered immediately after reconstitution. 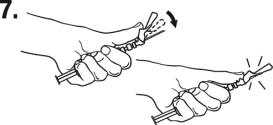
After administering the injection, immediately activate the safety mechanism by centering your thumb or forefinger on the textured finger pad area of the safety cover and pushing it forward over the needle until you hear or feel it lock. Use
the one-handed technique and activate the mechanism away from yourself and others. Activation of the safety cover causes
virtually no splatter. Immediately discard the syringe assembly after a single use into a suitable sharps container. - 3 DOSAGE FORMS AND STRENGTHS
-
4
CONTRAINDICATIONS
4.1 Hypersensitivity
TRELSTAR is contraindicated in individuals with a known hypersensitivity to triptorelin or any other component of the product, or other GnRH agonists or GnRH [see Warnings and Precautions (5.1)].
-
5
WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Anaphylactic shock, hypersensitivity, and angioedema related to triptorelin administration have been reported. In the event of a hypersensitivity reaction, therapy with TRELSTAR should be discontinued immediately and the appropriate supportive and symptomatic care should be administered.
5.2 Transient Increase in Serum Testosterone
Initially, triptorelin, like other GnRH agonists, causes a transient increase in serum testosterone levels. As a result, isolated cases of worsening of signs and symptoms of prostate cancer during the first weeks of treatment have been reported with GnRH agonists. Patients may experience worsening of symptoms or onset of new symptoms, including bone pain, neuropathy, hematuria, or urethral or bladder outlet obstruction [see Clinical Pharmacology (12.2)].
5.3 Metastatic Vertebral Lesions and Urinary Tract Obstruction
Cases of spinal cord compression, which may contribute to weakness or paralysis with or without fatal complications, have been reported with GnRH agonists. If spinal cord compression or renal impairment develops, standard treatment of these complications should be instituted, and in extreme cases an immediate orchiectomy considered.
Patients with metastatic vertebral lesions and/or with upper or lower urinary tract obstruction should be closely observed during the first few weeks of therapy.
5.4 Effect on QT/QTc Interval
Androgen deprivation therapy may prolong the QT/QTc interval. Providers should consider whether the benefits of androgen deprivation therapy outweigh the potential risks in patients with congenital long QT syndrome, congestive heart failure, frequent electrolyte abnormalities, and in patients taking drugs known to prolong the QT interval. Electrolyte abnormalities should be corrected. Consider periodic monitoring of electrocardiograms and electrolytes.
5.5 Hyperglycemia and Diabetes
Hyperglycemia and an increased risk of developing diabetes have been reported in men receiving GnRH agonists. Hyperglycemia may represent development of diabetes mellitus or worsening of glycemic control in patients with diabetes. Monitor blood glucose and/or glycosylated hemoglobin (HbA1c) periodically in patients receiving a GnRH agonist and manage with current practice for treatment of hyperglycemia or diabetes.
5.6 Cardiovascular Diseases
Increased risk of developing myocardial infarction, sudden cardiac death and stroke has been reported in association with use of GnRH agonists in men. The risk appears low based on the reported odds ratios, and should be evaluated carefully along with cardiovascular risk factors when determining a treatment for patients with prostate cancer. Patients receiving a GnRH agonist should be monitored for symptoms and signs suggestive of development of cardiovascular disease and be managed according to current clinical practice.
5.7 Laboratory Tests
Response to TRELSTAR should be monitored by measuring serum levels of testosterone periodically or as indicated.
5.8 Laboratory Test Interactions
Chronic or continuous administration of triptorelin in therapeutic doses results in suppression of pituitary-gonadal axis. Diagnostic tests of the pituitary-gonadal function conducted during treatment and after cessation of therapy may therefore be misleading.
5.9 Embryo-Fetal Toxicity
Based on findings from animal studies and mechanism of action, TRELSTAR can cause fetal harm when administered to a pregnant woman [Clinical Pharmacology (12.1)]. In animal developmental and reproductive toxicology studies, daily administration of triptorelin to pregnant rats during the period of organogenesis caused maternal toxicity and embryo-fetal toxicities, including loss of pregnancy, at doses as low as 0.2, 0.8, and 8 times the estimated human daily dose based on body surface area. Advise pregnant patients and females of reproductive potential of the potential risk to the fetus [see Use in Specific Populations (8.1)].
-
6
ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of the three TRELSTAR formulations was evaluated in clinical trials involving patients with advanced prostate cancer. Mean testosterone levels increased above baseline during the first week following the initial injection, declining thereafter to baseline levels or below by the end of the second week of treatment. The transient increase in testosterone levels may be associated with temporary worsening of disease signs and symptoms, including bone pain, neuropathy, hematuria, and urethral or bladder outlet obstruction. Isolated cases of spinal cord compression with weakness or paralysis of the lower extremities have occurred [see Warnings and Precautions (5.3)].
Adverse reactions reported for each of the three TRELSTAR formulations in the clinical trials, are presented in Table 2, Table 3, and Table 4. Often, causality is difficult to assess in patients with metastatic prostate cancer. The majority of adverse reactions related to triptorelin are a result of its pharmacological action, i.e., the induced variation in serum testosterone levels, either an increase in testosterone at the initiation of treatment, or a decrease in testosterone once castration is achieved. Local reactions at the injection site or allergic reactions may occur.
The following adverse reactions were reported to have a possible or probable relationship to therapy as ascribed by the treating physician in at least 1% of patients receiving TRELSTAR 3.75 mg.
Table 2. TRELSTAR 3.75 mg: Treatment-Related Adverse Reactions Reported by 1% or More of Patients During Treatment Adverse Reactions* TRELSTAR 3.75 mg
N = 140N % Application Site Disorders Injection site pain 5 3.6
Body as a WholeHot flush 82 58.6 Pain 3 2.1 Leg pain 3 2.1 Fatigue 3 2.1
Cardiovascular DisordersHypertension 5 3.6
Central and Peripheral Nervous System DisordersHeadache 7 5.0 Dizziness 2 1.4
Gastrointestinal DisordersDiarrhea 2 1.4 Vomiting 3 2.1
Musculoskeletal System DisordersSkeletal pain 17 12.1
Psychiatric DisordersInsomnia 3 2.1 Impotence 10 7.1 Emotional lability 2 1.4
Red Blood Cell DisordersAnemia 2 1.4
Skin and Appendages DisordersPruritus 2 1.4
Urinary System DisordersUrinary tract infection 2 1.4 Urinary retention 2 1.4 * Adverse reactions for TRELSTAR 3.75 mg are coded using the WHO Adverse Reactions Terminology (WHOART)
The following adverse reactions were reported to have a possible or probable relationship to therapy as ascribed by the treating physician in at least 1% of patients receiving TRELSTAR 11.25 mg.Table 3. TRELSTAR 11.25 mg: Treatment-Related Adverse Reactions Reported by 1% or More of Patients During Treatment
Adverse Reactions*TRELSTAR 11.25 mg
N = 174N %
Application SiteInjection site pain 7 4.0
Body as a WholeHot flush 127 73.0 Leg pain 9 5.2 Pain 6 3.4 Back pain 5 2.9 Fatigue 4 2.3 Chest pain 3 1.7 Asthenia 2 1.1 Peripheral edema 2 1.1
Cardiovascular DisordersHypertension 7 4.0 Dependent edema 4 2.3
Central and Peripheral Nervous System DisordersHeadache 12 6.9 Dizziness 5 2.9 Leg cramps 3 1.7
EndocrineBreast pain 4 2.3 Gynecomastia 3 1.7
Gastrointestinal DisordersNausea 5 2.9 Constipation 3 1.7 Dyspepsia 3 1.7 Diarrhea 2 1.1 Abdominal pain 2 1.1
Liver and Biliary SystemAbnormal hepatic function 2 1.1
Metabolic and Nutritional DisordersEdema in legs 11 6.3 Increased alkaline phosphatase 3 1.7
Musculoskeletal System DisordersSkeletal pain 23 13.2 Arthralgia 4 2.3 Myalgia 2 1.1
Psychiatric DisordersDecreased libido 4 2.3 Impotence 4 2.3 Insomnia 3 1.7 Anorexia 3 1.7
Respiratory System DisordersCoughing 3 1.7 Dyspnea 2 1.1 Pharyngitis 2 1.1
Skin and AppendagesRash 3 1.7
Urinary System DisordersDysuria 8 4.6 Urinary retention 2 1.1
Vision DisordersEye pain 2 1.1 Conjunctivitis 2 1.1 * Adverse reactions for TRELSTAR 11.25 mg are coded using the WHO Adverse Reactions Terminology (WHOART)
The following adverse reactions occurred in at least 5% of patients receiving TRELSTAR 22.5 mg. The table includes all reactions whether or not they were ascribed to TRELSTAR by the treating physician. The table also includes the incidence of these adverse reactions that were considered by the treating physician to have a reasonable causal relationship or for which the relationship could not be assessed.
Table 4. TRELSTAR 22.5 mg: Adverse Reactions Reported by 5% or More of Patients During Treatment
Adverse Reactions*TRELSTAR 22.5 mg
N = 120Treatment-Emergent Treatment-Related N % N %
General Disorders and Administration Site ConditionsEdema peripheral 6 5.0 0 0
Infections and InfestationsInfluenza 19 15.8 0 0 Bronchitis 6 5.0 0 0
EndocrineDiabetes Mellitus/Hyperglycemia 6 5.0 0 0
Musculoskeletal and Connective Tissue DisordersBack pain 13 10.8 1 0.8 Arthralgia 9 7.5 1 0.8 Pain in extremity 9 7.5 1 0.8
Nervous System DisordersHeadache 9 7.5 2 1.7
Psychiatric DisordersInsomnia 6 5.0 1 0.8
Renal and Urinary DisordersUrinary tract infection 14 11.6 0 0 Urinary retention 6 5.0 0 0
Reproductive System and Breast DisordersErectile dysfunction 12 10.0 12 10.0 Testicular atrophy 9 7.5 9 7.5
Vascular DisordersHot flush 87 72.5 86 71.7 Hypertension 17 14.2 1 0.8 * Adverse reactions for TRELSTAR 22.5 mg are coded using the Medical Dictionary for Regulatory Activities (MedDRA)
Changes in Laboratory Values During TreatmentThe following abnormalities in laboratory values not present at baseline were observed in 10% or more of patients:
TRELSTAR 3.75 mg: There were no clinically meaningful changes in laboratory values detected during therapy.
TRELSTAR 11.25 mg: Decreased hemoglobin and RBC count and increased glucose, BUN, SGOT, SGPT, and alkaline phosphatase at the Day 253 visit.
TRELSTAR 22.5 mg: Decreased hemoglobin and increased glucose and hepatic transaminases were detected during the study. The majority of the changes were mild to moderate.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of gonadotropin releasing hormone agonists. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
During postmarketing surveillance, rare cases of pituitary apoplexy (a clinical syndrome secondary to infarction of the pituitary gland) have been reported after the administration of gonadotropin-releasing hormone agonists. In a majority of these cases, a pituitary adenoma was diagnosed with a majority of pituitary apoplexy cases occurring within 2 weeks of the first dose, and some within the first hour. In these cases, pituitary apoplexy has presented as sudden headache, vomiting, visual changes, ophthalmoplegia, altered mental status, and sometimes cardiovascular collapse. Immediate medical attention has been required.
During postmarketing experience, convulsions, interstitial lung disease, and thromboembolic events including, but not limited to, pulmonary emboli, cerebrovascular accident, myocardial infarction, deep venous thrombosis, transient ischemic attack, and thrombophlebitis have been reported.
-
7
DRUG INTERACTIONS
No drug-drug interaction studies involving triptorelin have been conducted.
Human pharmacokinetic data with triptorelin suggest that C-terminal fragments produced by tissue degradation are either degraded completely within tissues or are rapidly degraded further in plasma, or cleared by the kidneys. Therefore, hepatic microsomal enzymes are unlikely to be involved in triptorelin metabolism. However, in the absence of relevant data and as a precaution, hyperprolactinemic drugs should not be used concomitantly with triptorelin since hyperprolactinemia reduces the number of pituitary GnRH receptors.
-
8
USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings in animal studies and mechanism of action, TRELSTAR can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. Expected hormonal changes that occur with TRELSTAR treatment increase the risk for pregnancy loss. In animal developmental and reproductive toxicology studies, daily administration of triptorelin to pregnant rats during the period of organogenesis caused maternal toxicity and embryo-fetal toxicities, including loss of pregnancy, at doses as low as 0.2, 0.8, and 8 times the estimated human daily dose based on body surface area. Advise pregnant patients and females of reproductive potential of the potential risk to the fetus.
Data
Animal Data
Studies in pregnant rats administered triptorelin at doses of 2, 10, and 100 mcg/kg/day (approximately equivalent to 0.2, 0.8, and 8 times the estimated human daily dose based on body surface area) during the period of organogenesis demonstrated maternal toxicity and embryo-fetal toxicities. Embryo-fetal toxicities consisted of pre-implantation loss, increased resorption, and reduced mean number of viable fetuses at the high dose. Teratogenic effects were not observed in viable fetuses in rats or mice. Doses administered to mice were 2, 20, and 200 mcg/kg/day (approximately equivalent to 0.1, 0.7, and 7 times the estimated human daily dose based on body surface area).
8.2 Lactation
The safety and efficacy of TRELSTAR have not been established in females. There are no data on the presence of triptorelin in human milk, the effects of the drug on milk production, or the effects of the drug on the breastfed child. Because of the potential for serious adverse reactions in a breastfed child from TRELSTAR, a decision should be made to either discontinue breastfeeding, or discontinue the drug taking into account the importance of the drug to the mother.
8.3 Females and Males of Reproductive Potential
Infertility
Males
Based on mechanism of action, TRELSTAR may impair fertility in males of reproductive potential [see Clinical Pharmacology (12.1)].
8.5 Geriatric Use
Prostate cancer occurs primarily in an older population. Clinical studies with TRELSTAR have been conducted primarily in patients ≥ 65 years [see Clinical Pharmacology (12.3) and Clinical Studies (14)].
8.6 Renal Impairment
Subjects with renal impairment had higher exposure than young healthy males [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Subjects with hepatic impairment had higher exposure than young healthy males [see Clinical Pharmacology (12.3)].
-
10
OVERDOSAGE
There is no experience of overdosage in clinical trials. In single dose toxicity studies in mice and rats, the subcutaneous LD50 of triptorelin was 400 mg/kg in mice and 250 mg/kg in rats, approximately 500 and 600 times, respectively, the estimated monthly human dose based on body surface area. If overdosage occurs, therapy should be discontinued immediately and the appropriate supportive and symptomatic treatment administered.
-
11
DESCRIPTION
TRELSTAR is a white to slightly yellow lyophilized cake. When reconstituted, TRELSTAR has a milky appearance. It contains a pamoate salt of triptorelin, a synthetic decapeptide agonist analog of gonadotropin releasing hormone (GnRH). The chemical name of triptorelin pamoate is 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-D-tryptophyl-L-leucyl-L-arginyl-L-prolylglycine amide (pamoate salt). The empirical formula is C64H82N18O13 · C23H16O6 and the molecular weight is 1699.9. The structural formula is:
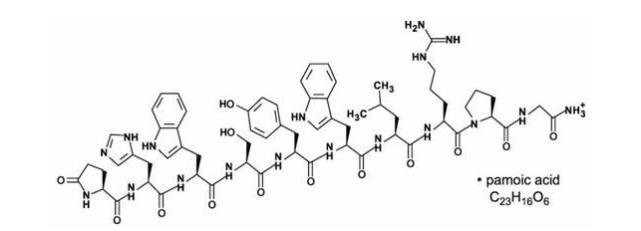
Structural formula for TRELSTAR (triptorelin pamoate).
The TRELSTAR products are sterile, lyophilized biodegradable microgranule formulations supplied as single dose vials. Refer to Table 5 for the composition of each TRELSTAR product.
Table 5. TRELSTAR Composition
IngredientsTRELSTAR
3.75 mgTRELSTAR
11.25 mgTRELSTAR
22.5 mg
triptorelin pamoate
(base units)3.75 mg 11.25 mg 22.5 mg
poly-d,l-lactide-co-glycolide138 mg 120 mg 183 mg
mannitol, USP71 mg 74 mg 74 mg
carboxymethylcellulose sodium, USP25 mg 26 mg 26 mg
polysorbate 80, NF1.7 mg 1.7 mg 1.7 mg When 2 mL sterile water is added to the vial containing TRELSTAR and mixed, a suspension is formed which is intended as an intramuscular injection. TRELSTAR is available in a vial plus a MIXJECT vial adapter, and a separate pre-filled syringe that contains sterile water for injection, USP, 2 mL, pH 6 to 8.5.
-
12
CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Triptorelin is a synthetic decapeptide agonist analog of gonadotropin releasing hormone (GnRH). Comparative in vitro studies showed that triptorelin was 100-fold more active than native GnRH in stimulating luteinizing hormone release from monolayers of dispersed rat pituitary cells in culture and 20-fold more active than native GnRH in displacing 125I-GnRH from pituitary receptor sites. In animal studies, triptorelin pamoate was found to have 13‑fold higher luteinizing hormone-releasing activity and 21-fold higher follicle-stimulating hormone-releasing activity compared to the native GnRH.
12.2 Pharmacodynamics
Following the first administration, there is a transient surge in circulating levels of luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone, and estradiol [see Adverse Reactions (6)]. After chronic and continuous administration, usually 2 to 4 weeks after initiation of therapy, a sustained decrease in LH and FSH secretion and marked reduction of testicular steroidogenesis are observed. A reduction of serum testosterone concentration to a level typically seen in surgically castrated men is obtained. Consequently, the result is that tissues and functions that depend on these hormones for maintenance become quiescent. These effects are usually reversible after cessation of therapy.
Following a single intramuscular injection of TRELSTAR:
TRELSTAR 3.75 mg: serum testosterone levels first increased, peaking on Day 4, and declined thereafter to low levels by Week 4 in healthy male volunteers.
TRELSTAR 11.25 mg: serum testosterone levels first increased, peaking on Days 2 – 3, and declined thereafter to low levels by Weeks 3 – 4 in men with advanced prostate cancer.
TRELSTAR 22.5 mg: serum testosterone levels first increased, peaking on Day 3, and declined thereafter to low levels by Weeks 3 – 4 in men with advanced prostate cancer.
12.3 Pharmacokinetics
Results of pharmacokinetic investigations conducted in healthy men indicate that after intravenous bolus administration, triptorelin is distributed and eliminated according to a 3-compartment model and corresponding half-lives are approximately 6 minutes, 45 minutes, and 3 hours.
Absorption
Following a single intramuscular injection of TRELSTAR to patients with prostate cancer, mean peak serum concentrations of 28.4 ng/mL, 38.5 ng/mL, and 44.1 ng/mL occurred in 1 to 3 hours after the 3.75 mg, 11.25 mg, and 22.5 mg formulations, respectively.
Triptorelin did not accumulate over 9 months (3.75 mg and 11.25 mg) or 12 months (22.5 mg) of treatment.
Distribution
The volume of distribution following a single intravenous bolus dose of 0.5 mg of triptorelin peptide was 30 – 33 L in healthy male volunteers. There is no evidence that triptorelin, at clinically relevant concentrations, binds to plasma proteins.
Elimination
Metabolism
The metabolism of triptorelin in humans is unknown, but is unlikely to involve hepatic microsomal enzymes (cytochrome P-450). The effect of triptorelin on the activity of other drug metabolizing enzymes is also unknown. Thus far, no metabolites of triptorelin have been identified. Pharmacokinetic data suggest that C-terminal fragments produced by tissue degradation are either completely degraded in the tissues, or rapidly degraded in plasma, or cleared by the kidneys.
Excretion
Triptorelin is eliminated by both the liver and the kidneys. Following intravenous administration of 0.5 mg triptorelin peptide to six healthy male volunteers with a creatinine clearance of 149.9 mL/min, 41.7% of the dose was excreted in urine as intact peptide with a total triptorelin clearance of 211.9 mL/min. This percentage increased to 62.3% in patients with liver disease who have a lower creatinine clearance (89.9 mL/min). It has also been observed that the nonrenal clearance of triptorelin (patient anuric, CIcreat = 0) was 76.2 mL/min, thus indicating that the nonrenal elimination of triptorelin is mainly dependent on the liver.
Special Populations
Age and Race
The effects of age and race on triptorelin pharmacokinetics have not been systematically studied. However, pharmacokinetic data obtained in young healthy male volunteers aged 20 to 22 years with an elevated creatinine clearance (approximately 150 mL/min) indicate that triptorelin was eliminated twice as fast in this young population as compared with patients with moderate renal insufficiency. This is related to the fact that triptorelin clearance is partly correlated to total creatinine clearance, which is well known to decrease with age [see Use in Specific Populations (8.6) and (8.7)].
Pediatric
TRELSTAR has not been evaluated in patients less than 18 years of age [see Use in Specific Populations (8.4)].
Hepatic and Renal Impairment
After an intravenous bolus injection of 0.5 mg triptorelin, the two distribution half-lives were unaffected by renal and hepatic impairment. However, renal insufficiency led to a decrease in total triptorelin clearance proportional to the decrease in creatinine clearance as well as increases in volume of distribution and consequently, an increase in elimination half-life (see Table 6). In subjects with hepatic insufficiency, a decrease in triptorelin clearance was more pronounced than that observed with renal insufficiency. Due to minimal increases in the volume of distribution, the elimination half-life in subjects with hepatic insufficiency was similar to subjects with renal insufficiency. Subjects with renal or hepatic impairment had 2‑ to 4-fold higher exposure (AUC values) than young healthy males [see Use in Specific Populations (8.6) and (8.7)].
Table 6. Pharmacokinetic Parameters (Mean ± SD) in Healthy Volunteers and Special Populations Following an IV Bolus Injection of 0.5 mg Triptorelin
GroupCmax
(ng/mL)AUCinf
(h·ng/mL)Clp
(mL/min)Clrenal
(mL/min)t1/2
(h)Clcreat
(mL/min)
6 healthy male volunteers48.2
±11.836.1
±5.8211.9
±31.690.6
±35.32.81
±1.21149.9
±7.3
6 males with moderate renal impairment45.6
±20.569.9
±24.6120.0
±45.023.3
±17.66.56
±1.2539.7
±22.5
6 males with severe renal impairment46.5
±14.088.0
±18.488.6
±19.74.3
±2.97.65
±1.258.9
±6.0
6 males with liver disease54.1
±5.3131.9
±18.157.8
±8.035.9
±5.07.58
±1.1789.9
±15.1 -
13
NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In rats, triptorelin doses of 120, 600, and 3000 mcg/kg given every 28 days (approximately 0.3, 2, and 8 times the human monthly dose based on body surface area) resulted in increased mortality with a drug treatment period of 13 – 19 months. The incidences of benign and malignant pituitary tumors and histiosarcomas were increased in a dose-related manner. No oncogenic effect was observed in mice administered triptorelin for 18 months at doses up to 6000 mcg/kg every 28 days (approximately 8 times the human monthly dose based on body surface area).
Mutagenicity studies performed with triptorelin using bacterial and mammalian systems (in vitro Ames test and chromosomal aberration test in CHO cells and an in vivo mouse micronucleus test) provided no evidence of mutagenic potential.
After 60 days of subcutaneous treatment followed by a minimum of four estrus cycles prior to mating, triptorelin, at doses of 2, 20, and 200 mcg/kg/day in saline (approximately 0.2, 2, and 16 times the estimated human daily dose based on body surface area) or 2 monthly injections as slow release microspheres (~20 mcg/kg/day), had no effect on the fertility or general reproductive function of female rats.
No studies were conducted to assess the effect of triptorelin on male fertility.
-
14
CLINICAL STUDIES
TRELSTAR 3.75 mg
TRELSTAR 3.75 mg was studied in a randomized, active control trial of 277 men with advanced prostate cancer. The clinical trial population consisted of 59.9% Caucasian, 39.3% Black, and 0.8% Other. There was no difference observed with triptorelin response between racial groups. Men were between 47 and 89 years of age (mean = 71 years). Patients received either TRELSTAR 3.75 mg (N = 140) or an approved GnRH agonist monthly for 9 months. The primary efficacy endpoints were both achievement of castration by Day 29 and maintenance of castration from Day 57 through Day 253.
Castration levels of serum testosterone (≤ 1.735 nmol/L; equivalent to 50 ng/dL) in patients treated with TRELSTAR 3.75 mg were achieved at Day 29 in 125 of 137 (91.2%) patients and at Day 57 in 97.7% of patients. Maintenance of castration levels of serum testosterone from Day 57 through Day 253 was found in 96.2% of patients treated with TRELSTAR 3.75 mg.
The presence of an acute-on-chronic flare phenomenon was also studied as a secondary efficacy endpoint. Serum LH levels were measured at 2 hours after repeat TRELSTAR 3.75 mg administration on Days 85 and 169. One hundred twenty-four of the 126 evaluable patients (98.4%) on Day 85 had a serum LH level of ≤ 1.0 IU/L at 2 hours after dosing, indicating desensitization of the pituitary gonadotroph receptors.
TRELSTAR 11.25 mg
TRELSTAR 11.25 mg was studied in a randomized, active control trial of 346 men with advanced prostate cancer. The clinical trial population consisted of 48% Caucasian, 38% Black, and 15% Other. There was no difference observed with triptorelin response between racial groups. Men were between 45 and 96 years of age (mean = 71 years). Patients received either TRELSTAR 11.25 mg (N = 174) every 12 weeks for a total of up to 3 doses (maximum treatment period of 253 days) or TRELSTAR 3.75 mg (N = 172) every 28 days for a total of up to 9 doses. The primary efficacy endpoints were both achievement of castration by Day 29 and maintenance of castration from Day 57 through Day 253.
Castration levels of serum testosterone (≤ 1.735 nmol/L; equivalent to 50 ng/dL) were achieved at Day 29 in 167 of 171 (97.7%) patients treated with TRELSTAR 11.25 mg, and maintenance of castration levels of serum testosterone from Day 57 through Day 253 was found in 94.4% of patients treated with TRELSTAR 11.25 mg.
TRELSTAR 22.5 mg
TRELSTAR 22.5 mg was studied in a non-comparative trial of 120 men with advanced prostate cancer. The clinical trial population consisted of 64% Caucasian, 23% Black, and 13% Other, with a mean age of 71.1 years (range 51-93). Patients received TRELSTAR 22.5 mg (N = 120) every 24 weeks for a total of 2 doses (maximum treatment period of 337 days). The primary efficacy endpoints included achievement of castration by Day 29 and maintenance of castration from Day 57 through Day 337.
Castration levels of serum testosterone (≤ 1.735 nmol/L; equivalent to 50 ng/dL) were achieved at Day 29 in 97.5% (117 of 120) of patients treated with TRELSTAR 22.5 mg. Castration was maintained in 93.3% of patients in the period from Day 57 to Day 337.
A summary of the clinical studies for TRELSTAR is provided in Table 7.
Table 7. Summary of TRELSTAR Clinical Studies
Product
Strength3.75 mg 11.25 mg 22.5 mg
Number of
Patients137 171 120
Treatment
Scheduleevery 4 weeks every 12 weeks every 24 weeks
Duration of Study253 days 253 days 337 days
Castration Rate*
on Day 29, %
(n/N)91.2% (125/137) 97.7% (167/171) 97.5% (117/120)
Rate of
Castration
Maintenance†
from Days 57 –
253, %96.2% 94.4% not applicable
Rate of
Castration
Maintenance
from Days 57 –
337, % (n/N)not applicable not applicable 93.3% (112/120)‡ * Maintenance of castration was calculated using a frequency distribution.
† Cumulative maintenance of castration was calculated using a survival analysis (Kaplan-Meier) technique.
‡ Calculation includes 5 patients who discontinued the study but who had castrate levels of testosterone prior to discontinuation. -
16
HOW SUPPLIED/STORAGE AND HANDLING
TRELSTAR is supplied in the TRELSTAR MIXJECT single-dose delivery system consisting of a vial with a Flip-Off seal containing sterile lyophilized triptorelin pamoate microgranules incorporated in a biodegradable copolymer of lactic and glycolic acids, a MIXJECT vial adapter, and a pre-filled syringe containing sterile water for injection, USP, 2 mL, pH 6 to 8.5.
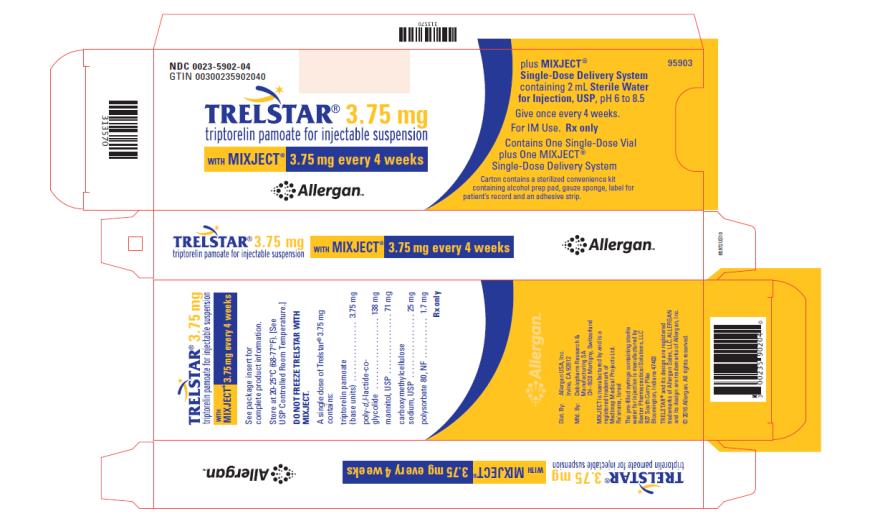
TRELSTAR 3.75 mg –NDC: 0023-5902-04 (TRELSTAR 3.75 mg with MIXJECT single-dose delivery system)
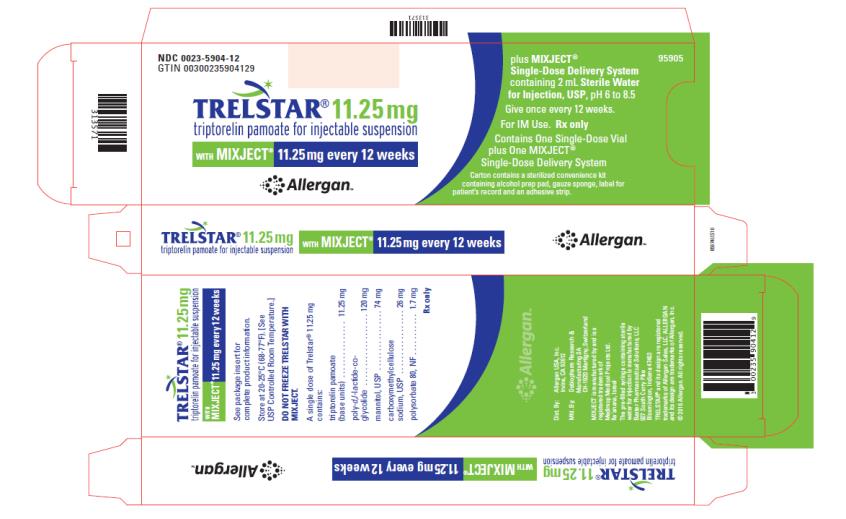
TRELSTAR 11.25 mg –NDC: 0023-5904-12 (TRELSTAR 11.25 mg with MIXJECT single-dose delivery system)
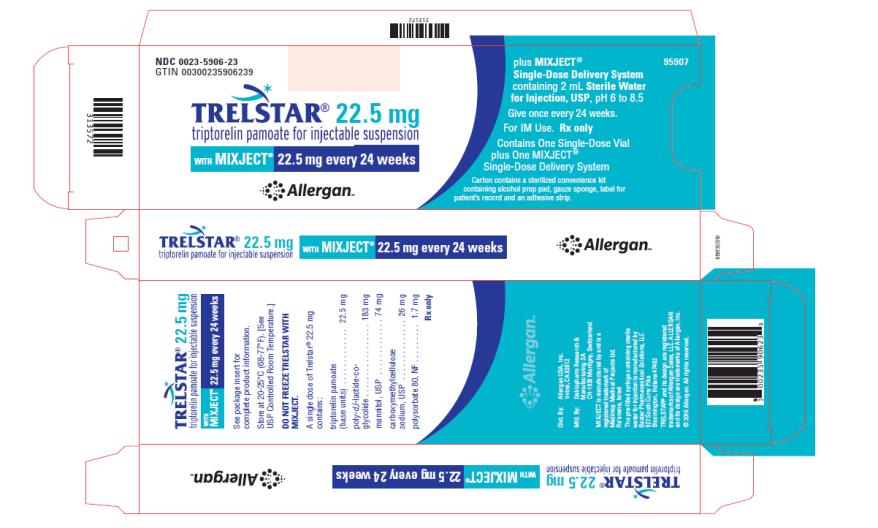
TRELSTAR 22.5 mg –NDC: 0023-5906-23 (TRELSTAR 22.5 mg with MIXJECT single-dose delivery system)
Storage
Store at 20-25°C (68-77°F). [See USP Controlled Room Temperature.] Do not freeze TRELSTAR with MIXJECT. -
17
PATIENT COUNSELING INFORMATION
Hypersensitivity
- Inform patients that if they have experienced hypersensitivity with other GnRH agonist drugs like TRELSTAR, TRELSTAR is contraindicated [see Contraindications (4)].
Tumor Flare
- Inform patients that TRELSTAR can cause tumor flare during the first weeks of treatment. Inform patients that the increase in testosterone can cause an increase in urinary symptoms or pain. Advise patients to contact their healthcare provider if uretral obstruction, spinal cord compression, paralysis, or new or worsened symptoms occur after beginning TRELSTAR treatment [see Warnings and Precautions (5.2)].
Hyperglycemia and Diabetes
- Advise patients that there is an increased risk of hyperglycemia and diabetes with TRELSTAR therapy. Inform patients that periodic monitoring for hyperglycemia and diabetes is required when being treated with TRELSTAR [see Warnings and Precautions (5.5)].
Cardiovascular Disease
- Inform patients that there is an increased risk of myocardial infarction, sudden cardiac death, and stroke with TRELSTAR treatment. Advise patients to immediately report signs and symptoms associated with these events to their healthcare provider for evaluation [see Warnings and Precautions (5.6)].
Urogenital Disorders
- Advise patients that TRELSTAR may cause impotence.
Infertility
- Inform patients that TRELSTAR may cause infertility [(see Use In Specific Populations (8.3)].
Continuation of TRELSTAR Treatment
- Inform patients that TRELSTAR is usually continued, often with additional medication, after the development of metastatic castration-resistant prostate cancer [see Dosage and Administration (2.1)].
For all medical inquiries contact:
Allergan
Medical Communications
1-800-678-1605Distributed By:
Allergan USA, Inc.
Madison, NJ 07940Manufactured By:
Debiopharm Research & Manufacturing SA
CH-1920 Martigny, SwitzerlandMIXJECT is manufactured by:
West Pharma. Services IL, Ltd.
Ra'anana, IsraelThe pre-filled syringe containing sterile water for injection is manufactured by:
Baxter Pharmaceutical Solutions, LLC
927 South Curry Pike
Bloomington, Indiana 47403TRELSTAR® and its design are registered trademarks of Allergan Sales, LLC
MIXJECT® is a registered trademark of West Pharma. Services IL, Ltd.
© 2018 Allergan. All rights reserved.
v2.0USPI5902
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TRELSTAR
triptorelin pamoate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0023-5902 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0023-5902-04 1 in 1 CARTON; Type 0: Not a Combination Product 06/15/2000 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-DOSE 2 mL Part 2 1 SYRINGE 2 mL Part 1 of 2 TRELSTAR
triptorelin pamoate injection, powder, lyophilized, for suspensionProduct Information Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIPTORELIN PAMOATE (UNII: 08AN7WA2G0) (TRIPTORELIN - UNII:9081Y98W2V) TRIPTORELIN 3.75 mg in 2 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020715 06/15/2000 Part 2 of 2 DILUENT
diluent liquidProduct Information Route of Administration INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 SYRINGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020715 06/15/2000 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020715 06/15/2000 TRELSTAR
triptorelin pamoate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0023-5904 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0023-5904-12 1 in 1 CARTON; Type 0: Not a Combination Product 06/29/2001 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-DOSE 2 mL Part 2 1 SYRINGE 2 mL Part 1 of 2 TRELSTAR
triptorelin pamoate injection, powder, lyophilized, for suspensionProduct Information Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIPTORELIN PAMOATE (UNII: 08AN7WA2G0) (TRIPTORELIN - UNII:9081Y98W2V) TRIPTORELIN 11.25 mg in 2 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021288 06/29/2001 Part 2 of 2 DILUENT
diluent liquidProduct Information Route of Administration INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 SYRINGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021288 06/29/2001 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021288 06/29/2001 TRELSTAR
triptorelin pamoate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0023-5906 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0023-5906-23 1 in 1 CARTON; Type 0: Not a Combination Product 03/11/2010 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-DOSE 2 mL Part 2 1 SYRINGE 2 mL Part 1 of 2 TRELSTAR
triptorelin pamoate injection, powder, lyophilized, for suspensionProduct Information Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIPTORELIN PAMOATE (UNII: 08AN7WA2G0) (TRIPTORELIN - UNII:9081Y98W2V) TRIPTORELIN 22.5 mg in 2 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022437 03/11/2010 Part 2 of 2 DILUENT
diluent liquidProduct Information Route of Administration INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 SYRINGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022437 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022437 03/11/2010 Labeler - Allergan, Inc. (144796497)
Trademark Results [Trelstar]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TRELSTAR 90201626 not registered Live/Pending |
Debiopharm Research & Manufacturing SA 2020-09-22 |
 TRELSTAR 78976890 3099457 Live/Registered |
ALLERGAN SALES, LLC 2004-12-15 |
 TRELSTAR 78533031 not registered Dead/Abandoned |
ALLERGAN SALES, LLC 2004-12-15 |
 TRELSTAR 78120194 2887476 Live/Registered |
ALLERGAN SALES, LLC 2002-04-08 |
 TRELSTAR 75638913 not registered Dead/Abandoned |
PHARMACIA & UPJOHN COMPANY 1999-02-10 |
 TRELSTAR 75636922 not registered Dead/Abandoned |
PHARMACIA & UPJOHN COMPANY 1999-02-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.