F10 Antiseptic Barrier Ointment with Insecticide 500g
F10 by
Drug Labeling and Warnings
F10 by is a Animal medication manufactured, distributed, or labeled by Health and Hygiene (Pty) Ltd, Lonza Group AG. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
F10- f10 antiseptic barrier ointment with insecticide ointment
Health and Hygiene (Pty) Ltd
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
F10 Antiseptic Barrier Ointment with Insecticide 500g
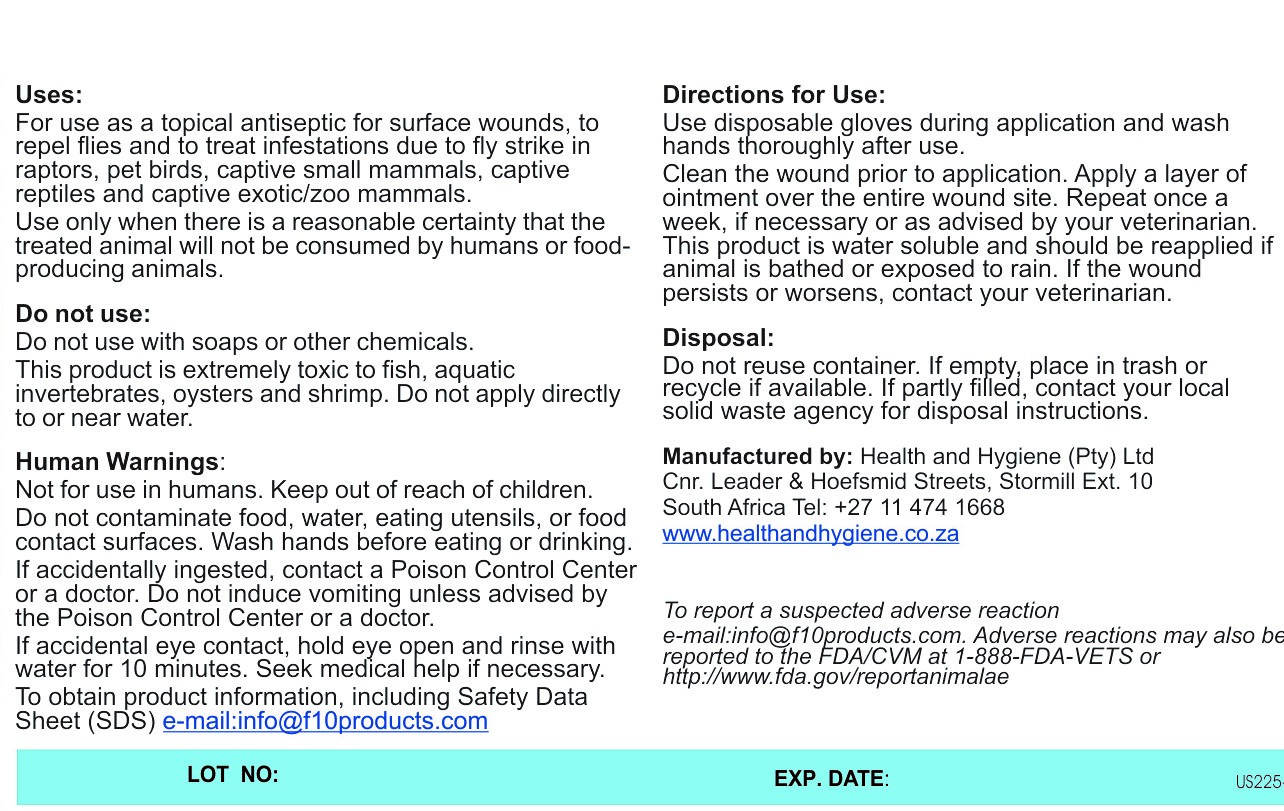
Uses
For use as a topical antiseptic for surface wounds, to repel flies and to treat infestations due to fly strike
| F10
f10 antiseptic barrier ointment with insecticide ointment |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Health and Hygiene (Pty) Ltd (636762007) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Health and Hygiene (Pty) Ltd | 636762007 | manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Lonza Group AG | 480007517 | api manufacture | |
Revised: 1/2022
Document Id: be7f9e40-995b-441d-a795-85e11fa56445
Set id: 7653cd1c-c20a-4e20-8672-837bd7812fab
Version: 2
Effective Time: 20220126