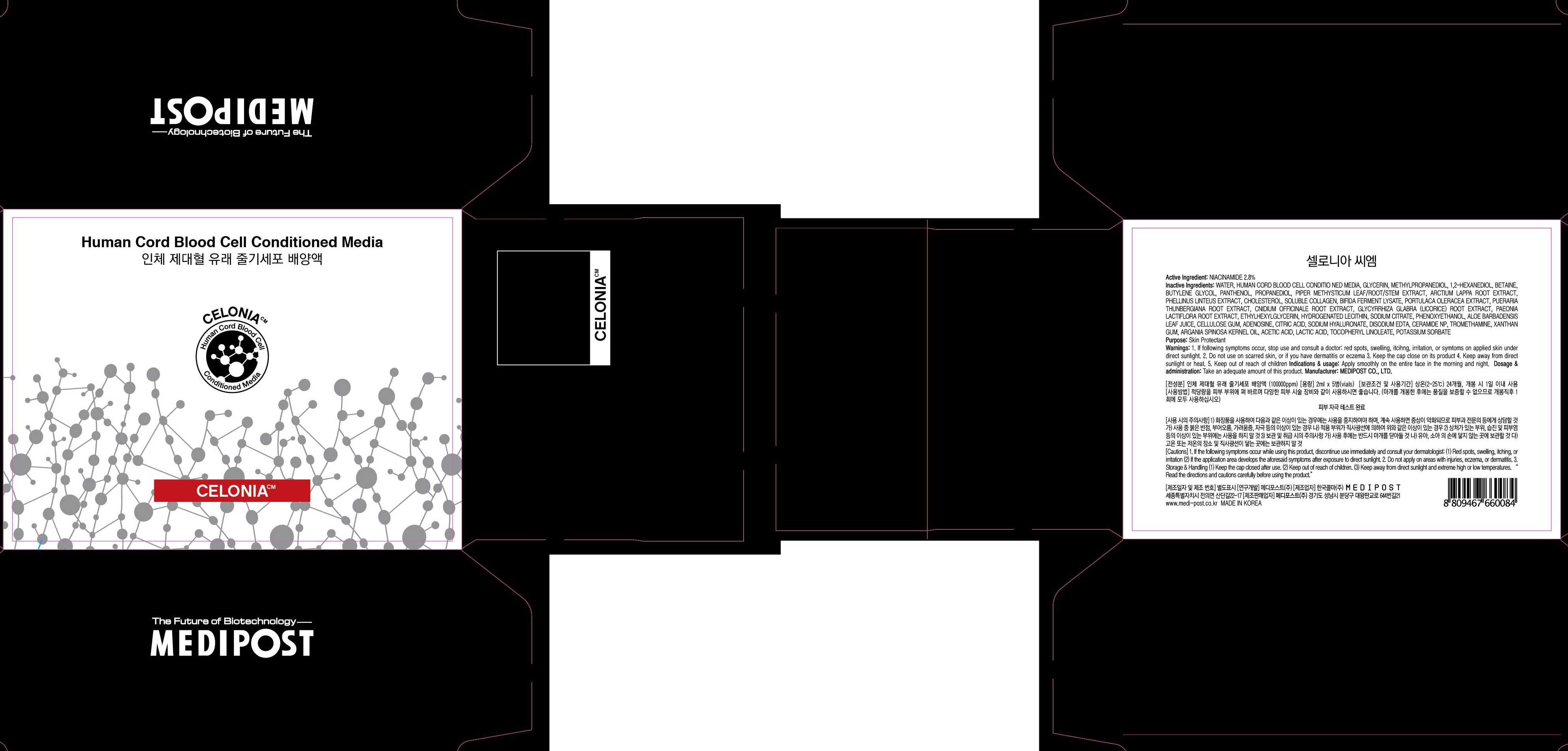
CELONIA CM- niacinamide solution
Celonia CM by
Drug Labeling and Warnings
Celonia CM by is a Otc medication manufactured, distributed, or labeled by MEDIPOST Co.,Ltd., Kolmar Korea Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Inactive Ingredients: WATER, HUMAN CORD BLOOD CELL CONDITIO NED MEDIA, GLYCERIN, METHYLPROPANEDIOL, 1,2-HEXANEDIOL, BETAINE, BUTYLENE GLYCOL, PANTHENOL, PROPANEDIOL, PIPER METHYSTICUM LEAF/ROOT/STEM EXTRACT, ARCTIUM LAPPA ROOT EXTRACT, PHELLINUS LINTEUS EXTRACT, CHOLESTEROL, SOLUBLE COLLAGEN, BIFIDA FERMENT LYSATE, PORTULACA OLERACEA EXTRACT, PUERARIA THUNBERGIANA ROOT EXTRACT, CNIDIUM OFFICINALE ROOT EXTRACT, GLYCYRRHIZA GLABRA (LICORICE) ROOT EXTRACT, PAEONIA LACTIFLORA ROOT EXTRACT, ETHYLHEXYLGLYCERIN, HYDROGENATED LECITHIN, SODIUM CITRATE, PHENOXYETHANOL, ALOE BARBADENSIS LEAF JUICE, CELLULOSE GUM, ADENOSINE, CITRIC ACID, SODIUM HYALURONATE, DISODIUM EDTA, CERAMIDE NP, TROMETHAMINE, XANTHAN GUM, ARGANIA SPINOSA KERNEL OIL, ACETIC ACID, LACTIC ACID, TOCOPHERYL LINOLEATE, POTASSIUM SORBATE
- PURPOSE
-
WARNINGS
Warnings: 1. If following symptoms occur, stop use and consult a doctor: red spots, swelling, itcihng, irritation, or symtoms on applied skin under direct sunlight. 2. Do not use on scarred skin, or if you have dermatitis or eczema 3. Keep the cap close on its product 4. Keep away from direct sunlight or heat. 5. Keep out of reach of children
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CELONIA CM
niacinamide solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 70506-120 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 0.05 g in 5 Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70506-120-01 5 in 1 CARTON; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/01/2016 Labeler - MEDIPOST Co.,Ltd. (688268817) Registrant - MEDIPOST Co.,Ltd. (688268817) Establishment Name Address ID/FEI Business Operations MEDIPOST Co.,Ltd. 688268817 repack(70506-120) Establishment Name Address ID/FEI Business Operations Kolmar Korea Co., Ltd. 689512611 manufacture(70506-120)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.