CALCIUM CARBONATE 1250 MG- calcium carbonate tablet
Calcium Carbonate by
Drug Labeling and Warnings
Calcium Carbonate by is a Otc medication manufactured, distributed, or labeled by CitraGen Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
-
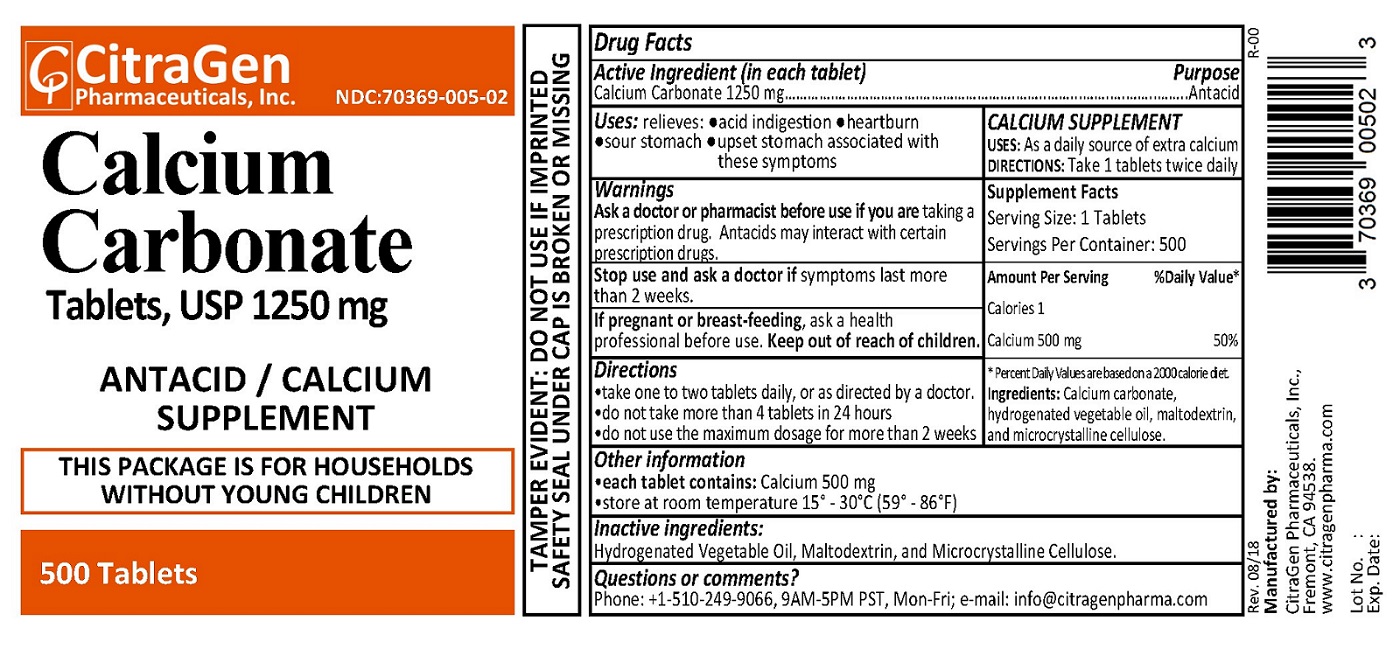
PRINCIPAL DISPLAY PANEL
CitraGen Pharmaceuticals, Inc.
NDC: 70369-005-02
Rev. 08/18 R-00
Calcium Carbonate Tablets, USP 1250 mg
ANTACID / Calcium Supplement
500 Tablets
THIS PACKAGE IS FOR HOUSEHOLDS WITHOUT YOUNG CHILDREN
-
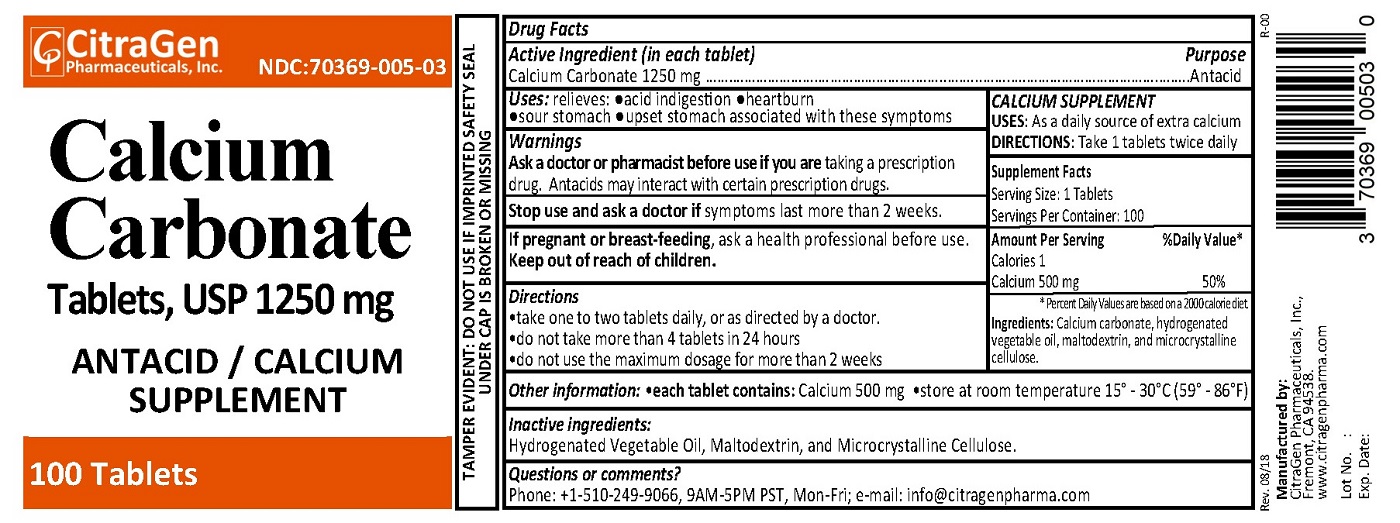
PRINCIPAL DISPLAY PANEL
CitraGen Pharmaceuticals, Inc.
NDC: 70369-005-03
Rev. 08/18 R-00
Calcium Carbonate Tablets, USP 1250 mg
ANTACID / Calcium Supplement
100 Tablets
-
INGREDIENTS AND APPEARANCE
CALCIUM CARBONATE 1250 MG
calcium carbonate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 70369-005 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 1250 mg Inactive Ingredients Ingredient Name Strength HYDROGENATED COTTONSEED OIL (UNII: Z82Y2C65EA) MALTODEXTRIN (UNII: 7CVR7L4A2D) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) Product Characteristics Color white Score no score Shape CAPSULE Size 18mm Flavor Imprint Code CG005 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70369-005-02 500 in 1 BOTTLE; Type 0: Not a Combination Product 09/26/2018 2 NDC: 70369-005-03 100 in 1 BOTTLE; Type 0: Not a Combination Product 09/26/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 09/26/2018 Labeler - CitraGen Pharmaceuticals, Inc. (024949457) Registrant - CitraGen Pharmaceuticals, Inc. (024949457) Establishment Name Address ID/FEI Business Operations CitraGen Pharmaceuticals, Inc. 024949457 manufacture(70369-005)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.