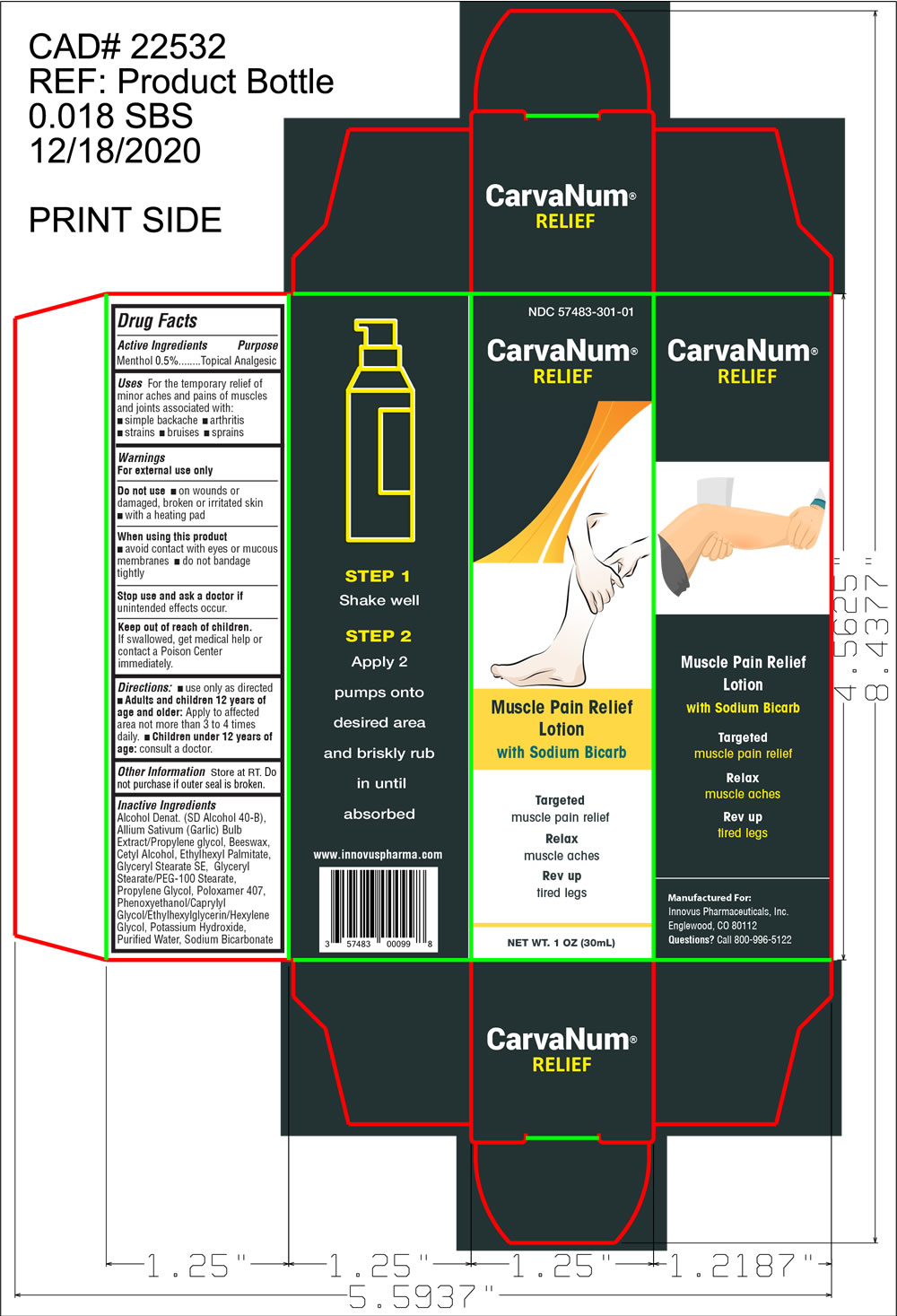
CarvaNum by Innovus Pharmaceuticals, Inc. CarvaNum
CarvaNum by
Drug Labeling and Warnings
CarvaNum by is a Otc medication manufactured, distributed, or labeled by Innovus Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CARVANUM- menthol lotion
Innovus Pharmaceuticals, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
CarvaNum
Uses
For the temporary relief minor aches and pains of muscles and joints associated with:
- simple backaches
- arthritis
- strains
- bruises
- sprains
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Center immediately
Directions
Use only as directed
- Adults and children 12 years of age and older: Apply to affected area not more than 3 to 4 times daily.
- Children under 12 years of age: consult a doctor
Inactive Ingredients
Alcohol Denat. (SD Alcohol 40-B), Allium Sativum (Garlic) Bulb Extract/Propylene glycol, Beeswax, Cetyl Alcohol, Ethylhexyl Palmitate, Glyceryl Stearate SE, Glyceryl Stearate/PEG-100 Stearate, Propylene Glycol, Poloxamer 407, Phenoxyethanol/Caprylyl Glycol/Ethylhexylglycerin/Hexylene Glycol, Potassium Hydroxide, Purified Water, Sodium Bicarbonate
| CARVANUM
menthol lotion |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Innovus Pharmaceuticals, Inc. (962507187) |
| Registrant - Innovus Pharmaceuticals, Inc. (962507187) |
Trademark Results [CarvaNum]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CARVANUM 88040646 not registered Live/Pending |
Innovus Pharmaceuticals, Inc. 2018-07-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.