LOW DOSE ASPIRIN by CARDINAL HEALTH / TIME CAP LABORATORIES, INC 481R - LEADER
LOW DOSE ASPIRIN by
Drug Labeling and Warnings
LOW DOSE ASPIRIN by is a Otc medication manufactured, distributed, or labeled by CARDINAL HEALTH, TIME CAP LABORATORIES, INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
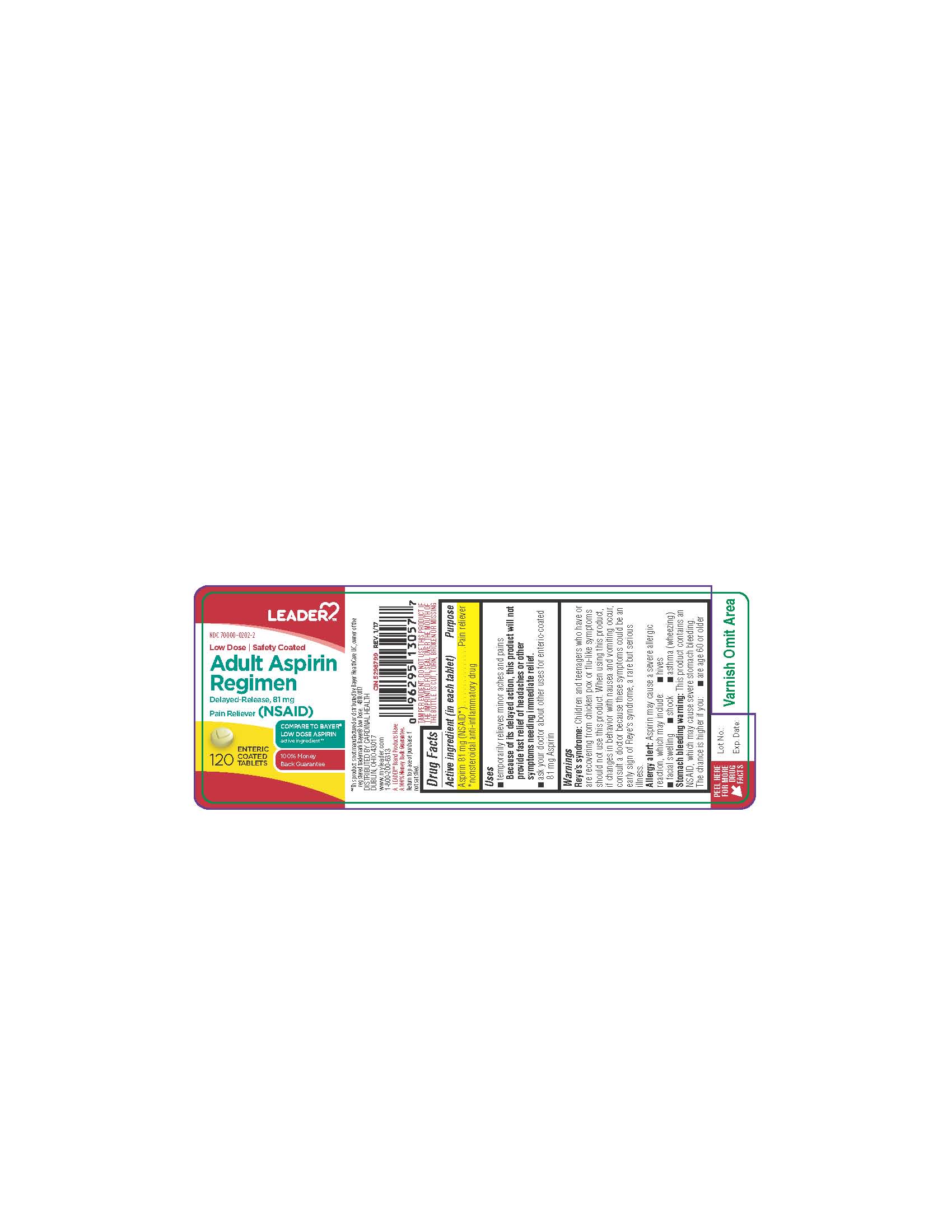
LOW DOSE ASPIRIN- aspirin tablet, coated
CARDINAL HEALTH
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
481R - LEADER
|
Active ingredient (in each tablet) Purpose Aspirin 81 mg (NSAID*). . . . . . . . . . . . . . .Pain reliever *nonsteroidal anti-inflammatory drug |
|
anhydrous lactose, carnauba wax, colloidal silicon dioxide, croscarmellose sodium, D&C yellow #10 aluminum lake, iron oxide ochre, methacrylic acid and ethyl acrylate copolymer, microcrystalline cellulose, polysorbate 80, simethicone, sodium hydroxide, sodium lauryl sulfate, starch, talc, titanium dioxide, triethyl citrate |
|
Directions _ drink a full glass of water with each dose _ adults and children 12 years and over: take 4 to 8 tablets every 4 hours not to exceed 48 tablets in 24 hours unless directed by a doctor _ children under 12 years: consult a doctor |
|
Uses _ temporarily relieves minor aches and pains Because of its delayed action, this product will not provide fast relief of headaches or other symptoms needing immediate relief. |
|
Warnings Reye’s syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness. Allergy alert: Aspirin may cause a severe allergic reaction, which may include: _ hives _ facial swelling _ shock _ asthma (wheezing) Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you: _ are age 60 or olde |
r
| LOW DOSE ASPIRIN
aspirin tablet, coated |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - CARDINAL HEALTH (097537435) |
| Registrant - TIME CAP LABORATORIES, INC (037052099) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TIME CAP LABORATORIES, INC | 037052099 | manufacture(70000-0202) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.