GOOD NEIGHBOR PHARMACY DAYHIST ALLERGY- clemastine fumarate tablet
Good Neighbor Pharmacy Dayhist Allergy by
Drug Labeling and Warnings
Good Neighbor Pharmacy Dayhist Allergy by is a Otc medication manufactured, distributed, or labeled by Amerisource Bergen. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
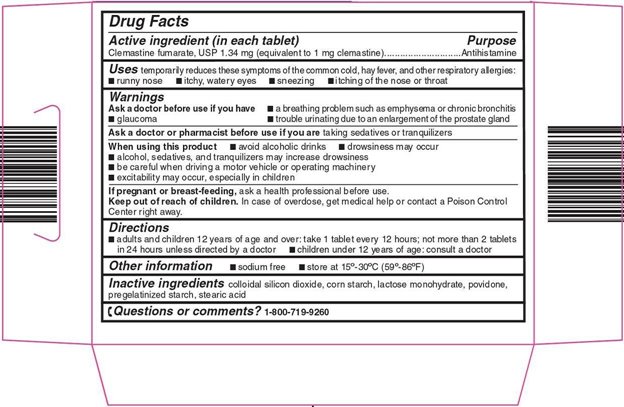
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlargement of the prostate gland
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
GOOD NEIGHBOR PHARMACY DAYHIST ALLERGY
clemastine fumarate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 24385-183 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLEMASTINE FUMARATE (UNII: 19259EGQ3D) (CLEMASTINE - UNII:95QN29S1ID) CLEMASTINE FUMARATE 1.34 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) STEARIC ACID (UNII: 4ELV7Z65AP) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color WHITE (off white) Score 2 pieces Shape CAPSULE Size 9mm Flavor Imprint Code L282 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 24385-183-51 1 in 1 CARTON 06/28/1996 1 8 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074512 06/28/1996 Labeler - Amerisource Bergen (007914906)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.