TETRACAINE HYDROCHLORIDE solution
Tetracaine Hydrochloride by
Drug Labeling and Warnings
Tetracaine Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by NuCare Pharmaceuticals,Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION:
Tetracaine Hydrochloride is a sterile aqueous topical anesthetic ophthalmic solution. The active ingredient is represented by the chemical structure:
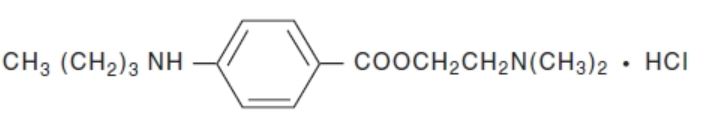
C 15H 24N 2O 2·HCI
Mol. wt. 300.83Benzoic acid, 4-[butylamino]-, 2-[dimethylamino]ethyl ester, monohydrochloride.
EACH mL CONTAINS: ACTIVE: Tetracaine Hydrochloride 5 mg (0.5%); INACTIVES: Boric Acid, Potassium Chloride, Edetate Disodium and Purified Water. Sodium Hydroxide and/or Hydrochloric Acid may be added to adjust pH (3.7 - 6.0). PRESERVATIVE ADDED: Chlorobutanol 0.4%.
- CLINICAL PHARMACOLOGY:
- INDICATIONS AND USAGE:
- CONTRAINDICATIONS:
- WARNINGS:
- PRECAUTIONS:
- NOTE:
-
ADVERSE REACTIONS:
Transient symptoms (signs) such as stinging, burning and conjunctival redness may occur. A rare, severe, immediate allergic cornea reaction has been reported, characterized by acute diffuse epithelial keratitis with filament formation and/or sloughing of large areas of necrotic epithelium, diffuse stromal edema, descemetitis and iritis.
-
DOSAGE AND ADMINISTRATION:
For tonometry and other procedures of short duration, instill one or two drops just prior to evaluation. For minor surgical procedures such as foreign body or suture removal, administer one to two drops every five to ten minutes for one to three instillations. For prolonged anesthesia as in cataract extraction, instill one or two drops in the eye(s) every five to ten minutes for three to five doses.

-
HOW SUPPLIED:
Tetracaine Hydrochloride Ophthalmic Solution USP, 0.5% is supplied in a plastic bottle with a controlled drop tip in the following size:
NDC: 68071-4579-5 BOX OF 15mL
- STORAGE:
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TETRACAINE HYDROCHLORIDE
tetracaine hydrochloride solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68071-4579(NDC: 69292-317) Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TETRACAINE HYDROCHLORIDE (UNII: 5NF5D4OPCI) (TETRACAINE - UNII:0619F35CGV) TETRACAINE HYDROCHLORIDE 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) POTASSIUM CHLORIDE (UNII: 660YQ98I10) EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) CHLOROBUTANOL (UNII: HM4YQM8WRC) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68071-4579-5 15 mL in 1 BOX; Type 0: Not a Combination Product 09/27/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/31/2018 Labeler - NuCare Pharmaceuticals,Inc. (010632300) Establishment Name Address ID/FEI Business Operations NuCare Pharmaceuticals,Inc. 010632300 relabel(68071-4579)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
