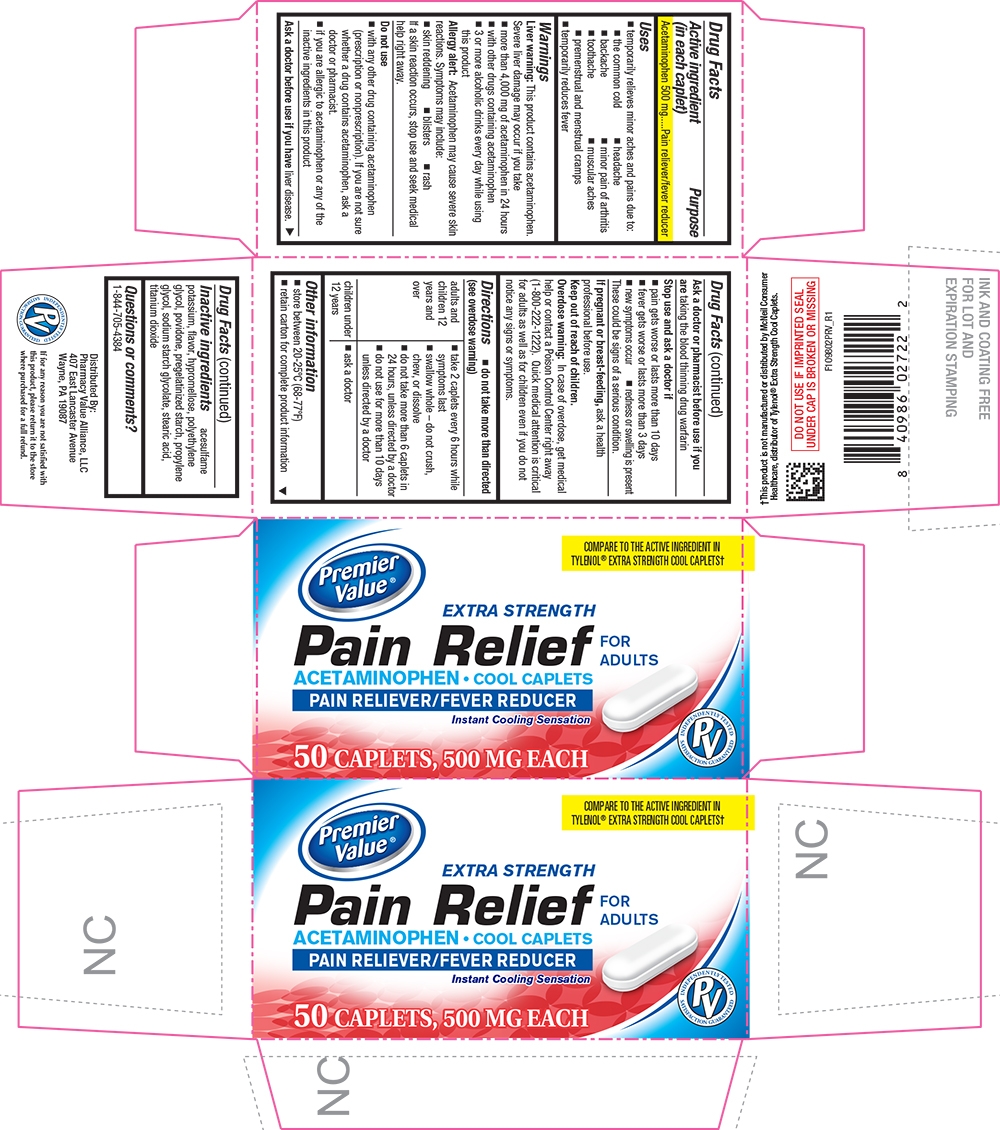
Pain Relief by CHAIN DRUG CONSORTIUM 1098-PRV-2020-0823
Pain Relief by
Drug Labeling and Warnings
Pain Relief by is a Otc medication manufactured, distributed, or labeled by CHAIN DRUG CONSORTIUM. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PAIN RELIEF- acetaminophen tablet, coated
CHAIN DRUG CONSORTIUM
----------
1098-PRV-2020-0823
Uses
- temporarily relieves minor aches and pains due to:
- the common cold
- headache
- backache
- minor pain of arthritis
- toothache
- muscular aches
- premenstrual and menstrual cramps
- temporarily reduces fever
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert
acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
Directions
- do not take more than directed (see overdose warning)
| adults and children 12 years and over |
|
| children under 12 years |
|
| PAIN RELIEF
acetaminophen tablet, coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - CHAIN DRUG CONSORTIUM (101668460) |
Revised: 10/2023
Document Id: 08f37d93-37ff-67f4-e063-6394a90a81d3
Set id: 770b437f-605b-4b16-8f46-acd2ead1c40d
Version: 4
Effective Time: 20231030
Trademark Results [Pain Relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PAIN RELIEF 97238381 not registered Live/Pending |
Liu, Caiqing 2022-01-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.