Epinephrine by Mylan Specialty L.P. EPINEPHRINE injection
Epinephrine by
Drug Labeling and Warnings
Epinephrine by is a Prescription medication manufactured, distributed, or labeled by Mylan Specialty L.P.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use EPINEPHRINE INJECTION safely and effectively. See full prescribing information for EPINEPHRINE INJECTION.
EPINEPHRINE injection, for intramuscular or subcutaneous use
Initial U.S. Approval: 1939INDICATIONS AND USAGE
Epinephrine injection, USP auto-injector contains epinephrine, a non-selective alpha and beta-adrenergic receptor agonist, indicated in the emergency treatment of allergic reactions (Type I) including anaphylaxis. (1)
DOSAGE AND ADMINISTRATION
- Patients greater than or equal to 30 kg (66 lbs): epinephrine injection, USP auto-injector, 0.3 mg (2)
- Patients 15 to 30 kg (33 lbs to 66 lbs): epinephrine injection, USP auto-injector, 0.15 mg (2)
Inject intramuscularly or subcutaneously into the anterolateral aspect of the thigh, through clothing if necessary. Each device is a single-use injection. (2)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
- In conjunction with use, seek immediate medical or hospital care. (5.1)
- Do not inject intravenously, into buttock, or into digits, hands, or feet. (5.2)
- To minimize the risk of injection related injury, instruct caregivers to hold the child’s leg firmly in place and limit movement prior to and during injection when administering to young children. (5.2)
- Rare cases of serious skin and soft tissue infections have been reported following epinephrine injection. Advise patients to seek medical care if they develop signs or symptoms of infection. (5.3)
- The presence of a sulfite in this product should not deter use. (5.4)
- Administer with caution in patients with heart disease; may aggravate angina pectoris or produce ventricular arrhythmias. (5.5)
ADVERSE REACTIONS
Adverse reactions to epinephrine include anxiety, apprehensiveness, restlessness, tremor, weakness, dizziness, sweating, palpitations, pallor, nausea and vomiting, headache, and/or respiratory difficulties. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Mylan at 1-877-446-3679 (1-877-4-INFO-RX) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Cardiac glycosides or diuretics: observe for development of cardiac arrhythmias. (7)
- Tricyclic antidepressants, monoamine oxidase inhibitors, levothyroxine sodium, and certain antihistamines: potentiate effects of epinephrine. (7)
- Beta-adrenergic blocking drugs: antagonize cardiostimulating and bronchodilating effects of epinephrine. (7)
- Alpha-adrenergic blocking drugs: antagonize vasoconstricting and hypertensive effects of epinephrine. (7)
- Ergot alkaloids: may reverse the pressor effects of epinephrine. (7)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 1/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Emergency Treatment
5.2 Injection-Related Complications
5.3 Serious Infections at the Injection Site
5.4 Allergic Reactions Associated with Sulfite
5.5 Disease Interactions
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Epinephrine injection, USP auto-injectors are indicated in the emergency treatment of allergic reactions (Type I) including anaphylaxis to stinging insects (e.g., order Hymenoptera, which include bees, wasps, hornets, yellow jackets and fire ants) and biting insects (e.g., triatoma, mosquitoes), allergen immunotherapy, foods, drugs, diagnostic testing substances (e.g., radiocontrast media) and other allergens, as well as idiopathic anaphylaxis or exercise-induced anaphylaxis.
Epinephrine injection, USP auto-injectors are intended for immediate administration in patients who are determined to be at increased risk for anaphylaxis, including individuals with a history of anaphylactic reactions.
Anaphylactic reactions may occur within minutes after exposure and consist of flushing, apprehension, syncope, tachycardia, thready or unobtainable pulse associated with a fall in blood pressure, convulsions, vomiting, diarrhea and abdominal cramps, involuntary voiding, wheezing, dyspnea due to laryngeal spasm, pruritus, rashes, urticaria or angioedema.
Epinephrine injection, USP auto-injectors are intended for immediate administration as emergency supportive therapy only and are not a substitute for immediate medical care.
-
2 DOSAGE AND ADMINISTRATION
Selection of the appropriate epinephrine injection, USP auto-injector is determined according to patient body weight.
- Patients greater than or equal to 30 kg (approximately 66 pounds or more): epinephrine injection, USP auto-injector, 0.3 mg
- Patients 15 kg to 30 kg (33 pounds to 66 pounds): epinephrine injection, USP auto-injector, 0.15 mg
Inject epinephrine injection, USP auto-injector intramuscularly or subcutaneously into the anterolateral aspect of the thigh, through clothing if necessary. Instruct caregivers of young children who are prescribed an epinephrine injection, USP auto-injector and who may be uncooperative and kick or move during an injection to hold the leg firmly in place and limit movement prior to and during an injection [see Warnings and Precautions (5.2)].
Each epinephrine injection, USP auto-injector contains a single dose of epinephrine for single-use injection. Since the doses of epinephrine delivered from epinephrine injection, USP auto-injector are fixed, consider using other forms of injectable epinephrine if doses lower than 0.15 mg are deemed necessary.
The prescriber should carefully assess each patient to determine the most appropriate dose of epinephrine, recognizing the life-threatening nature of the reactions for which this drug is indicated. With severe persistent anaphylaxis, repeat injections with an additional epinephrine injection, USP auto-injector may be necessary. More than two sequential doses of epinephrine should only be administered under direct medical supervision [see Warnings and Precautions (5.1)].
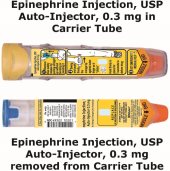
The epinephrine solution in the clear window of the epinephrine injection, USP auto-injector should be inspected visually for particulate matter and discoloration. Epinephrine is light sensitive and should be stored in the carrier tube provided to protect it from light [see How Supplied/Storage and Handling (16.2)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Emergency Treatment
Epinephrine injection, USP auto-injectors are intended for immediate administration as emergency supportive therapy and are not intended as a substitute for immediate medical care. In conjunction with the administration of epinephrine, the patient should seek immediate medical or hospital care. More than two sequential doses of epinephrine should only be administered under direct medical supervision [see Indications and Usage (1), Dosage and Administration (2) and Patient Counseling Information (17)].
5.2 Injection-Related Complications
Epinephrine injection, USP auto-injector should only be injected into the anterolateral aspect of the thigh [see Dosage and Administration (2) and Patient Counseling Information (17)].
- Do not inject intravenously. Large doses or accidental intravenous injection of epinephrine may result in cerebral hemorrhage due to sharp rise in blood pressure. Rapidly acting vasodilators can counteract the marked pressor effects of epinephrine if there is such inadvertent administration.
- Do not inject into buttock. Injection into the buttock may not provide effective treatment of anaphylaxis. Advise the patient to go immediately to the nearest emergency room for further treatment of anaphylaxis. Additionally, injection into the buttock has been associated with Clostridial infections (gas gangrene). Cleansing with alcohol does not kill bacterial spores, and therefore, does not lower this risk.
- Do not inject into digits, hands or feet. Since epinephrine is a strong vasoconstrictor, accidental injection into the digits, hands or feet may result in loss of blood flow to the affected area. Advise the patient to go immediately to the nearest emergency room and to inform the healthcare provider in the emergency room of the location of the accidental injection. Treatment of such inadvertent administration should consist of vasodilation, in addition to further appropriate treatment of anaphylaxis [see Adverse Reactions (6)].
- Hold leg firmly during injection. Lacerations, bent needles, and embedded needles have been reported when epinephrine injection, USP auto-injector has been injected into the thigh of young children who are uncooperative and kick or move during an injection. To minimize the risk of injection related injury when administering epinephrine injection, USP auto-injector to young children, instruct caregivers to hold the child’s leg firmly in place and limit movement prior to and during injection.
5.3 Serious Infections at the Injection Site
Rare cases of serious skin and soft tissue infections, including necrotizing fasciitis and myonecrosis caused by Clostridia (gas gangrene), have been reported at the injection site following epinephrine injection for anaphylaxis. Clostridium spores can be present on the skin and introduced into the deep tissue with subcutaneous or intramuscular injection. While cleansing with alcohol may reduce presence of bacteria on the skin, alcohol cleansing does not kill Clostridium spores. To decrease the risk of Clostridium infection, do not inject epinephrine injection, USP auto-injector into the buttock [see Warnings and Precautions (5.2)]. Advise patients to seek medical care if they develop signs or symptoms of infection, such as persistent redness, warmth, swelling, or tenderness, at the epinephrine injection site.
5.4 Allergic Reactions Associated with Sulfite
The presence of a sulfite in this product should not deter administration of the drug for treatment of serious allergic or other emergency situations even if the patient is sulfite-sensitive.
Epinephrine is the preferred treatment for serious allergic reactions or other emergency situations even though this product contains sodium metabisulfite, a sulfite that may, in other products, cause allergic-type reactions including anaphylactic symptoms or life-threatening or less severe asthmatic episodes in certain susceptible persons.
The alternatives to using epinephrine in a life-threatening situation may not be satisfactory.
5.5 Disease Interactions
Some patients may be at greater risk for developing adverse reactions after epinephrine administration. Despite these concerns, it should be recognized that the presence of these conditions is not a contraindication to epinephrine administration in an acute, life-threatening situation. Therefore, patients with these conditions, and/or any other person who might be in a position to administer epinephrine injection, USP auto-injector to a patient experiencing anaphylaxis should be carefully instructed in regard to the circumstances under which epinephrine should be used.
- Patients with Heart Disease
Epinephrine should be administered with caution to patients who have heart disease, including patients with cardiac arrhythmias, coronary artery or organic heart disease, or hypertension. In such patients, or in patients who are on drugs that may sensitize the heart to arrhythmias, epinephrine may precipitate or aggravate angina pectoris as well as produce ventricular arrhythmias [see Drug Interactions (7) and Adverse Reactions (6)].
- Other Patients and Diseases
Epinephrine should be administered with caution to patients with hyperthyroidism, diabetes, elderly individuals, and pregnant women. Patients with Parkinson’s disease may notice a temporary worsening of symptoms.
-
6 ADVERSE REACTIONS
Due to the lack of randomized, controlled clinical trials of epinephrine for the treatment of anaphylaxis, the true incidence of adverse reactions associated with the systemic use of epinephrine is difficult to determine. Adverse reactions reported in observational trials, case reports, and studies are listed below.
Common adverse reactions to systemically administered epinephrine include anxiety; apprehensiveness; restlessness; tremor; weakness; dizziness; sweating; palpitations; pallor; nausea and vomiting; headache; and/or respiratory difficulties. These symptoms occur in some persons receiving therapeutic doses of epinephrine, but are more likely to occur in patients with hypertension or hyperthyroidism [see Warnings and Precautions (5.5)].
Arrhythmias, including fatal ventricular fibrillation, have been reported, particularly in patients with underlying cardiac disease or those receiving certain drugs [see Warnings and Precautions (5.5) and Drug Interactions (7)].
Rapid rises in blood pressure have produced cerebral hemorrhage, particularly in elderly patients with cardiovascular disease [see Warnings and Precautions (5.5)].
Angina may occur in patients with coronary artery disease [see Warnings and Precautions (5.5)].
Rare cases of stress cardiomyopathy have been reported in patients treated with epinephrine.
Accidental injection into the digits, hands or feet may result in loss of blood flow to the affected area [see Warnings and Precautions (5.2)].
Adverse events experienced as a result of accidental injections may include increased heart rate, local reactions including injection site pallor, coldness and hypoesthesia or injury at the injection site resulting in bruising, bleeding, discoloration, erythema or skeletal injury.
Lacerations, bent needles, and embedded needles have been reported when epinephrine injection, USP auto-injector has been injected into the thigh of young children who are uncooperative and kick or move during the injection [see Warning and Precautions (5.2)].
Injection into the buttock has resulted in cases of gas gangrene [see Warnings and Precautions (5.2)].
Rare cases of serious skin and soft tissue infections, including necrotizing fasciitis and myonecrosis caused by Clostridia (gas gangrene), have been reported following epinephrine injection, including epinephrine injection, USP auto-injector, in the thigh [see Warnings and Precautions (5.3)].
-
7 DRUG INTERACTIONS
Patients who receive epinephrine while concomitantly taking cardiac glycosides, diuretics, or anti-arrhythmics should be observed carefully for the development of cardiac arrhythmias [see Warnings and Precautions (5.5)].
The effects of epinephrine may be potentiated by tricyclic antidepressants, monoamine oxidase inhibitors, levothyroxine sodium, and certain antihistamines, notably chlorpheniramine, tripelennamine, and diphenhydramine.
The cardiostimulating and bronchodilating effects of epinephrine are antagonized by beta- adrenergic blocking drugs, such as propranolol.
The vasoconstricting and hypertensive effects of epinephrine are antagonized by alpha- adrenergic blocking drugs, such as phentolamine.
Ergot alkaloids may also reverse the pressor effects of epinephrine.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well controlled studies of the acute effect of epinephrine in pregnant women. In animal reproductive studies, epinephrine administered by the subcutaneous route to rabbits, mice, and hamsters during the period of organogenesis was teratogenic at doses 7 times and higher than the maximum recommended human intramuscular and subcutaneous dose on a mg/m2 basis. Epinephrine is the first-line medication of choice for the treatment of anaphylaxis during pregnancy in humans. Epinephrine should be used for treatment of anaphylaxis during pregnancy in the same manner as it is used in non-pregnant patients.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-associated maternal and embryo/fetal risk
During pregnancy, anaphylaxis can be catastrophic and can lead to hypoxic-ischemic encephalopathy and permanent central nervous system damage or death in the mother and, more commonly, in the fetus or neonate. The prevalence of anaphylaxis occurring during pregnancy is reported to be approximately 3 cases per 100,000 deliveries.
Management of anaphylaxis during pregnancy is similar to management in the general population. Epinephrine is the first line-medication of choice for treatment of anaphylaxis; it should be used in the same manner in pregnant and non-pregnant patients. In conjunction with the administration of epinephrine, the patient should seek immediate medical or hospital care.
Data
Animal Data
In an embryofetal development study with rabbits dosed during the period of organogenesis, epinephrine was shown to be teratogenic (including gastroschisis and embryonic lethality) at doses approximately 40 times the maximum recommended intramuscular or subcutaneous dose (on a mg/m2 basis at a maternal subcutaneous dose of 1.2 mg/kg/day for two to three days).
In an embryofetal development study with mice dosed during the period of organogenesis, epinephrine was shown to be teratogenic (including embryonic lethality) at doses approximately 8 times the maximum recommended intramuscular or subcutaneous dose (on a mg/m2 basis at maternal subcutaneous dose of 1 mg/kg/day for 10 days). These effects were not seen in mice at approximately 4 times the maximum recommended daily intramuscular or subcutaneous dose (on a mg/m2 basis at a subcutaneous maternal dose of 0.5 mg/kg/day for 10 days).
In an embryofetal development study with hamsters dosed during the period of organogenesis from gestation days 7 to 10, epinephrine was shown to be teratogenic at doses approximately 7 times the maximum recommended intramuscular or subcutaneous dose (on a mg/m2 basis at a maternal subcutaneous dose of 0.5 mg/kg/day).
8.2 Lactation
Risk Summary
There is no information on the presence of epinephrine in human milk, the effects on breastfed infants, or the effects on milk production. Epinephrine is the first line-medication of choice for treatment of anaphylaxis; it should be used in the same manner in breastfeeding and non-breastfeeding patients.
8.4 Pediatric Use
Epinephrine injection, USP auto-injector may be administered to pediatric patients at a dosage appropriate to body weight [see Dosage and Administration (2)]. Clinical experience with the use of epinephrine suggests that the adverse reactions seen in children are similar in nature and extent to those both expected and reported in adults. Since the doses of epinephrine delivered from epinephrine injection, USP auto-injectors are fixed, consider using other forms of injectable epinephrine if doses lower than 0.15 mg are deemed necessary.
8.5 Geriatric Use
Clinical studies for the treatment of anaphylaxis have not been performed in subjects aged 65 and over to determine whether they respond differently from younger subjects. However, other reported clinical experience with use of epinephrine for the treatment of anaphylaxis has identified that geriatric patients may be particularly sensitive to the effects of epinephrine. Therefore, epinephrine injection, USP auto-injector should be administered with caution in elderly individuals, who may be at greater risk for developing adverse reactions after epinephrine administration [see Warnings and Precautions (5.5), Overdosage (10)].
-
10 OVERDOSAGE
Overdosage of epinephrine may produce extremely elevated arterial pressure, which may result in cerebrovascular hemorrhage, particularly in elderly patients. Overdosage may also result in pulmonary edema because of peripheral vascular constriction together with cardiac stimulation. Treatment consists of rapidly acting vasodilators or alpha-adrenergic blocking drugs and/or respiratory support.
Epinephrine overdosage can also cause transient bradycardia followed by tachycardia, and these may be accompanied by potentially fatal cardiac arrhythmias. Premature ventricular contractions may appear within one minute after injection and may be followed by multifocal ventricular tachycardia (prefibrillation rhythm). Subsidence of the ventricular effects may be followed by atrial tachycardia and occasionally by atrioventricular block. Treatment of arrhythmias consists of administration of a beta-adrenergic blocking drug such as propranolol.
Overdosage sometimes results in extreme pallor and coldness of the skin, metabolic acidosis, and kidney failure. Suitable corrective measures must be taken in such situations.
-
11 DESCRIPTION
Epinephrine injection, USP auto-injectors, 0.3 mg and 0.15 mg, are auto-injectors and combination products containing drug and device components.
Each epinephrine injection, USP auto-injector, 0.3 mg delivers a single dose of 0.3 mg epinephrine from epinephrine injection, USP 0.3 mg/0.3 mL in a sterile solution.
Each epinephrine injection, USP auto-injector, 0.15 mg delivers a single dose of 0.15 mg epinephrine from epinephrine injection, USP 0.15 mg/0.3 mL in a sterile solution.
The epinephrine injection, USP auto-injector contains 2 mL epinephrine solution. Approximately 1.7 mL remains in the auto-injector after activation, but is not available for future use, and should be discarded.
Each 0.3 mL in the epinephrine injection, USP auto-injector, 0.3 mg contains 0.3 mg epinephrine, 1.8 mg sodium chloride, 0.5 mg sodium metabisulfite, hydrochloric acid to adjust pH, and Water for Injection. The pH range is 2.2-5.0.
Each 0.3 mL in the epinephrine injection, USP auto-injector, 0.15 mg contains 0.15 mg epinephrine, 1.8 mg sodium chloride, 0.5 mg sodium metabisulfite, hydrochloric acid to adjust pH, and Water for Injection. The pH range is 2.2-5.0.
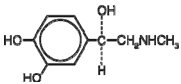
Epinephrine is a sympathomimetic catecholamine. Chemically, epinephrine is (-)-3,4- Dihydroxy-α-[(methylamino)methyl]benzyl alcohol with the following structure:
Epinephrine solution deteriorates rapidly on exposure to air or light, turning pink from oxidation to adrenochrome and brown from the formation of melanin. Replace epinephrine injection, USP auto-injector if the epinephrine solution appears discolored (pinkish or brown color), cloudy, or contains particles.
Thoroughly review the patient instructions and operation of epinephrine injection, USP auto-injector with patients and caregivers prior to use [see Patient Counseling Information (17)].
-
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
Through its action on alpha-adrenergic receptors, epinephrine lessens the vasodilation and increased vascular permeability that occurs during anaphylaxis, which can lead to loss of intravascular fluid volume and hypotension.
Through its action on beta-adrenergic receptors, epinephrine causes bronchial smooth muscle relaxation and helps alleviate bronchospasm, wheezing and dyspnea that may occur during anaphylaxis.
Epinephrine also alleviates pruritus, urticaria, and angioedema and may relieve gastrointestinal and genitourinary symptoms associated with anaphylaxis because of its relaxer effects on the smooth muscle of the stomach, intestine, uterus and urinary bladder.
When given subcutaneously or intramuscularly, epinephrine has a rapid onset and short duration of action.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies to evaluate the carcinogenic potential of epinephrine have not been conducted.
Epinephrine and other catecholamines have been shown to have mutagenic potential in vitro. Epinephrine was positive in the Salmonella bacterial reverse mutation assay, positive in the mouse lymphoma assay, and negative in the in vivo micronucleus assay. Epinephrine is an oxidative mutagen based on the E. coli WP2 Mutoxitest bacterial reverse mutation assay. This should not prevent the use of epinephrine under the conditions noted under Indications and Usage (1).
The potential for epinephrine to impair reproductive performance has not been evaluated, but epinephrine has been shown to decrease implantation in female rabbits dosed subcutaneously with 1.2 mg/kg/day (40-fold the highest human intramuscular or subcutaneous daily dose) during gestation days 3 to 9.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
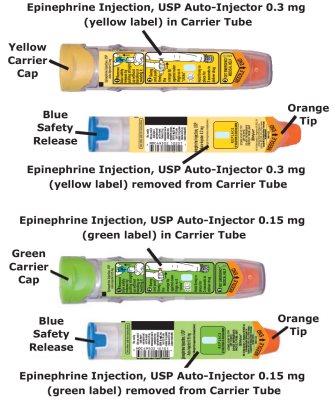
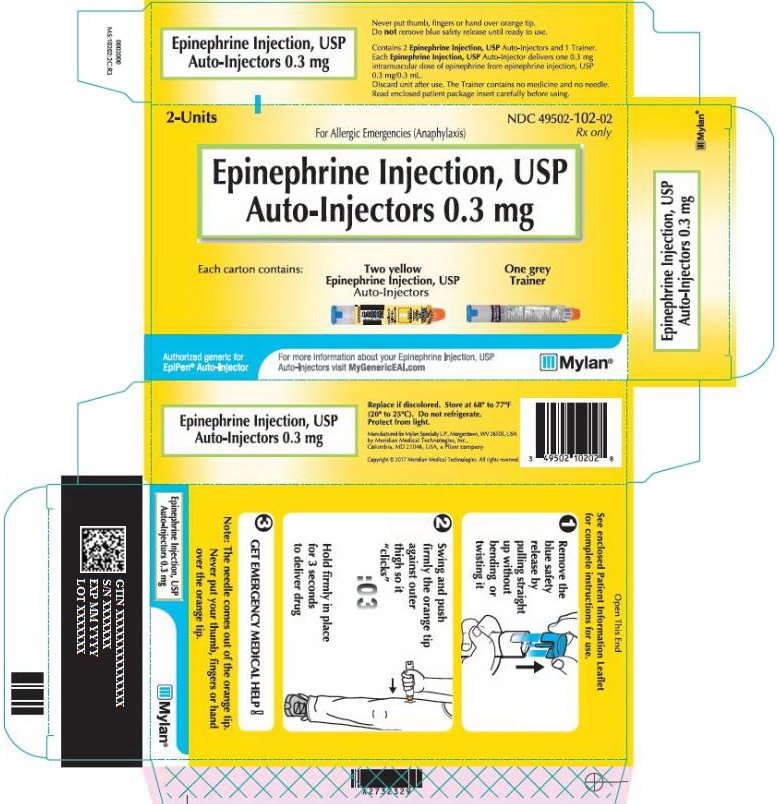
Epinephrine Injection, USP Auto-Injectors, 0.3 mg (epinephrine injections, USP 0.3 mg/0.3 mL) are available as NDC: 49502-102-02, a pack that contains two Epinephrine Injection, USP Auto-Injectors, 0.3 mg (epinephrine injections, USP 0.3 mg/0.3 mL) and one Epinephrine Injection, USP Auto-Injector Trainer device.
Epinephrine Injection, USP Auto-Injectors, 0.15 mg (epinephrine injections, USP 0.15 mg/0.3 mL) are available as NDC: 49502-101-02, a pack that contains two Epinephrine Injection, USP Auto-Injectors, 0.15 mg (epinephrine injections, USP 0.15 mg/0.3 mL) and one Epinephrine Injection, USP Auto-Injector Trainer device.
Epinephrine Injection, USP Auto-Injectors also include an S-clip to clip two carrier tubes together.
Rx only
16.2 Storage and Handling
Protect from light. Epinephrine is light sensitive and should be stored in the carrier tube provided to protect it from light. Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]. Do not refrigerate. Before using, check to make sure the solution in the auto-injector is clear and colorless. Replace the auto-injector if the solution is discolored (pinkish or brown color), cloudy, or contains particles. Properly dispose all used, unwanted or expired epinephrine injection, USP auto-injectors [see Patient Counseling Information (17)].
-
17 PATIENT COUNSELING INFORMATION
[See FDA-Approved Patient Labeling (Patient Information and Instructions for Use).]
A healthcare provider should review the patient instructions and operation of epinephrine injection, USP auto-injector in detail, with the patient or caregiver.
Epinephrine is essential for the treatment of anaphylaxis. Patients who are at risk of or with a history of severe allergic reactions (anaphylaxis) to insect stings or bites, foods, drugs, and other allergens, as well as idiopathic and exercise-induced anaphylaxis, should be carefully instructed about the circumstances under which epinephrine should be used.
Administration and Training
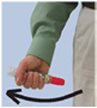
Instruct patients and/or caregivers in the appropriate use of epinephrine injection, USP auto-injector. Epinephrine injection, USP auto-injector should be injected into the middle of the outer thigh (through clothing, if necessary). Each device is a single-use injection. Advise patients to seek immediate medical care in conjunction with administration of epinephrine injection, USP auto-injectors.
Instruct caregivers to hold the leg of young children firmly in place and limit movement prior to and during injection. Lacerations, bent needles, and embedded needles have been reported when epinephrine injection, USP auto-injector has been injected into the thigh of young children who are uncooperative and kick or move during an injection [see Warnings and Precautions (5.2)].
Instruct patients and/or caregivers to throw away the blue safety release immediately after using epinephrine injection, USP auto-injector. This small part may pose a choking hazard for children.
Complete patient information, including dosage, directions for proper administration and precautions can be found inside each epinephrine injection, USP auto-injector carton. A printed label on the surface of epinephrine injection, USP auto-injector shows instructions for use and a diagram depicting the injection process.
Instruct patients and/or caregivers to use and practice with the Trainer to familiarize themselves with the use of epinephrine injection, USP auto-injector in an allergic emergency. The Trainer may be used multiple times. A Trainer device is provided in epinephrine injection, USP auto-injector cartons.
Instruct patients and/or caregivers to immediately place the blue safety release back on the Trainer and reset it after practicing. This small part may pose a choking hazard for children.
Adverse Reactions
Epinephrine may produce symptoms and signs that include an increase in heart rate, the sensation of a more forceful heartbeat, palpitations, sweating, nausea and vomiting, difficulty breathing, pallor, dizziness, weakness or shakiness, headache, apprehension, nervousness, or anxiety. These signs and symptoms usually subside rapidly, especially with rest, quiet and recumbency. Patients with hypertension or hyperthyroidism may develop more severe or persistent effects, and patients with coronary artery disease could experience angina. Patients with diabetes may develop increased blood glucose levels following epinephrine administration. Patients with Parkinson’s disease may notice a temporary worsening of symptoms [see Warnings and Precautions (5.5)].
Accidental Injection
Advise patients to seek immediate medical care in the case of accidental injection. Since epinephrine is a strong vasoconstrictor when injected into the digits, hands, or feet, treatment should be directed at vasodilatation if there is such an accidental injection to these areas [see Warnings and Precautions (5.2)].
Serious Infections at the Injection Site
Rare cases of serious skin and soft tissue infections, including necrotizing fasciitis and myonecrosis caused by Clostridia (gas gangrene), have been reported at the injection site following epinephrine injection for anaphylaxis. Advise patients to seek medical care if they develop signs or symptoms of infection, such as persistent redness, warmth, swelling, or tenderness, at the epinephrine injection site [see Warnings and Precautions (5.3)].
Storage and Handling
Instruct patients to inspect the epinephrine solution visually through the clear window of the auto-injector periodically. Replace epinephrine injection, USP auto-injector if the epinephrine solution appears discolored (pinkish or brown color), cloudy, or contains particles. Epinephrine is light sensitive and should be stored in the carrier tube provided to protect it from light. The carrier tube is not waterproof. Instruct patients that epinephrine injection, USP auto-injector must be used or properly disposed once the blue safety release is removed or after use [see Storage and Handling (16.2)].
Advise patients and caregivers to promptly dispose of medicines that are no longer needed. Dispose of expired, unwanted, or unused epinephrine injection, USP auto-injectors in an FDA-cleared sharps container. Instruct patients not to dispose epinephrine injection, USP auto-injectors in their household trash. Instruct patients that if they do not have a FDA-cleared sharps disposal container, they may use a household container that is made of a heavy-duty plastic, can be closed with a tight-fitting and puncture-resistant lid without sharps being able to come out, upright and stable during use, leak-resistant, and properly labeled to warn of hazardous waste inside the container. Inform patients that they can visit the FDA website for additional information on disposal of unused medicines.
Complete patient information, including dosage, directions for proper administration and precautions can be found inside each epinephrine injection, USP auto-injector carton.
Manufactured for Mylan Specialty L.P., Morgantown, WV 26505, U.S.A. by Meridian Medical Technologies, Inc., Columbia, MD 21046, U.S.A., a Pfizer company
Copyright © 2020 Meridian Medical Technologies. All rights reserved.
Revised: 1/2020
MS:EPIG:R4
0002076 -
PATIENT INFORMATION and INSTRUCTIONS FOR USE
Epinephrine Injection, USP Auto-Injector 0.3 mg
one dose of 0.3 mg epinephrine, USP 0.3 mg/0.3 mLEpinephrine Injection, USP Auto-Injector 0.15 mg
one dose of 0.15 mg epinephrine, USP 0.15 mg/0.3 mLAuthorized generic for EpiPen® and EpiPen Jr® Auto-Injectors
For allergic emergencies (anaphylaxis)
Patient Information
Read this Patient Information Leaflet carefully before using the epinephrine injection, USP auto-injector and each time you get a refill. There may be new information. You, your parent, caregiver, or others who may be in a position to administer epinephrine injection, USP auto-injector, should know how to use it before you have an allergic emergency.
This information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about the Epinephrine Injection, USP Auto-Injector?
- 1.
Epinephrine injection, USP auto-injector contains epinephrine, a medicine used to treat allergic emergencies (anaphylaxis). Anaphylaxis can be life threatening, can happen within minutes, and can be caused by stinging and biting insects, allergy injections, foods, medicines, exercise, or unknown causes.
Symptoms of anaphylaxis may include:- trouble breathing
- wheezing
- hoarseness (changes in the way your voice sounds)
- hives (raised reddened rash that may itch)
- severe itching
- swelling of your face, lips, mouth, or tongue
- skin rash, redness, or swelling
- fast heartbeat
- weak pulse
- feeling very anxious
- confusion
- stomach pain
- losing control of urine or bowel movements (incontinence)
- diarrhea or stomach cramps
- dizziness, fainting, or “passing out” (unconsciousness)
- 2.
Always carry your epinephrine injection, USP auto-injector with you because you may not know when anaphylaxis may happen.
Talk to your healthcare provider if you need additional units to keep at work, school, or other locations. Tell your family members, caregivers, and others where you keep your epinephrine injection, USP auto-injector and how to use it before you need it. You may be unable to speak in an allergic emergency. - 3.
When you have an allergic emergency (anaphylaxis)
- Use epinephrine injection, USP auto-injector right away.
- Get emergency medical help right away. You may need further medical attention. You may need to use a second epinephrine injection, USP auto-injector if symptoms continue or recur. Only a healthcare provider should give additional doses of epinephrine if you need more than 2 injections for a single anaphylaxis episode.
What are epinephrine injection, USP auto-injectors?
- Epinephrine injection, USP auto-injectors are disposable, prefilled automatic injection devices (auto-injectors) used to treat life-threatening, allergic emergencies including anaphylaxis in people who are at risk for or have a history of serious allergic emergencies. Each device contains a single dose of epinephrine.
- Epinephrine injection, USP auto-injectors are for immediate self (or caregiver) administration and do not take the place of emergency medical care. You should get emergency help right away after using epinephrine injection, USP auto-injector.
- Epinephrine injection, USP auto-injectors are for people who have been prescribed this medicine by their healthcare provider.
- The epinephrine injection, USP auto-injector (0.3 mg) is for patients who weigh 66 pounds or more (30 kilograms or more).
- The epinephrine injection, USP auto-injector (0.15 mg) is for patients who weigh about 33 to 66 pounds (15 to 30 kilograms).
- It is not known if epinephrine injection, USP auto-injector is safe and effective in children who weigh less than 33 pounds (15 kilograms).
What should I tell my healthcare provider before using the epinephrine injection, USP auto-injector?
Before you use epinephrine injection, USP auto-injector, tell your healthcare provider about all your medical conditions, but especially if you:
- have heart problems or high blood pressure.
- have diabetes.
- have thyroid problems.
- have asthma.
- have a history of depression.
- have Parkinson’s disease.
- are pregnant or plan to become pregnant. It is not known if epinephrine will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if epinephrine passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Tell your healthcare provider about all of your known allergies.
Especially tell your healthcare provider if you take certain asthma medicines.
Epinephrine injection, USP auto-injector and other medicines may affect each other, causing side effects. Epinephrine injection, USP auto-injector may affect the way other medicines work, and other medicines may affect how epinephrine injection, USP auto-injector works.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
Use your epinephrine injection, USP auto-injector for treatment of anaphylaxis as prescribed by your healthcare provider, regardless of your medical conditions or the medicines you take.
How should I use epinephrine injection, USP auto-injectors?
- Each epinephrine injection, USP auto-injector contains only 1 dose of medicine.
- Epinephrine injection, USP auto-injector should be injected into the middle of your outer thigh (upper leg). It can be injected through your clothing if needed.
- Read the Instructions for Use at the end of this Patient Information Leaflet about the right way to use epinephrine injection, USP auto-injectors.
- Your healthcare provider will show you how to safely use the epinephrine injection, USP auto-injector.
- Use your epinephrine injection, USP auto-injector exactly as your healthcare provider tells you to use it. You may need to use a second epinephrine injection, USP auto-injector if symptoms continue or recur. Only a healthcare provider should give additional doses of epinephrine if you need more than 2 injections for a single anaphylaxis episode.
- Caution: Never put your thumb, fingers, or hand over the orange tip. Never press or push the orange tip with your thumb, fingers, or hand. The needle comes out of the orange tip. Accidental injection into finger, hands or feet may cause a loss of blood flow to these areas. If this happens, go immediately to the nearest emergency room. Tell the healthcare provider where on your body you received the accidental injection.
- Your epinephrine injection, USP auto-injectors may come packaged with an epinephrine injection, USP auto-injector Trainer and separate Trainer Instructions for Use. The epinephrine injection, USP auto-injector Trainer has a grey color. The grey epinephrine injection, USP auto-injector Trainer contains no medicine and no needle. Periodically practice with your epinephrine injection, USP auto-injector Trainer before an allergic emergency happens to make sure you are able to safely use the real epinephrine injection, USP auto-injector in an emergency. Always carry your real epinephrine injection, USP auto-injector with you in case of an allergic emergency. Additional training resources are available at MyGenericEAI.com.
- Do not drop the carrier tube or auto-injector. If the carrier tube or auto-injector is dropped, check for damage and leakage. Throw away (dispose of) the auto-injector and carrier tube and replace if damage or leakage is noticed or suspected.
What are the possible side effects of epinephrine injection, USP auto-injectors?
Epinephrine injection, USP auto-injectors may cause serious side effects.
-
The epinephrine injection, USP auto-injector should only be injected into the middle of your outer thigh (upper leg).Do not inject the epinephrine injection, USP auto-injector into your:
- veins
- buttocks
-
fingers, toes, hands, or feet
If you accidentally inject epinephrine injection, USP auto-injector into any other part of your body, go to the nearest emergency room right away. Tell the healthcare provider where on your body you received the accidental injection.
-
Rarely, patients who have used epinephrine injection, USP auto-injector may develop infections at the injection site within a few days of an injection. Some of these infections can be serious. Call your healthcare provider right away if you have any of the following at an injection site:
- redness that does not go away
- swelling
- tenderness
- the area feels warm to the touch
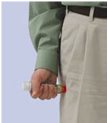
- Cuts on the skin, bent needles, and needles that remain in the skin after the injection, have happened in young children who do not cooperate and kick or move during an injection. If you inject a young child with epinephrine injection, USP auto-injector, hold their leg firmly in place before and during the injection to prevent injuries. Ask your healthcare provider to show you how to properly hold the leg of a young child during injection.
- If you have certain medical conditions, or take certain medicines, your condition may get worse or you may have longer lasting side effects when you use your epinephrine injection, USP auto-injector. Talk to your healthcare provider about all your medical conditions.
Common side effects of epinephrine injection, USP auto-injectors include:
- fast, irregular or “pounding” heartbeat
- sweating
- headache
- weakness
- shakiness
- paleness
- feelings of over excitement, nervousness or anxiety
- dizziness
- nausea or vomiting
- breathing problems
These side effects may go away with rest. Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of the epinephrine injection, USP auto-injector. For more information, ask your healthcare provider or pharmacist.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store epinephrine injection, USP auto-injectors?
- Store epinephrine injection, USP auto-injectors at room temperature between 68°F to 77°F (20°C to 25°C).
- Protect from light.
- Do not expose to extreme cold or heat. For example, do not store in your vehicle’s glove box and do not store in the refrigerator or freezer.
- Examine the contents in the clear window of your auto-injector periodically. The solution should be clear. If the solution is discolored (pinkish or brown color) or contains solid particles, replace the unit.
- Always keep your epinephrine injection, USP auto-injector in the carrier tube to protect it from damage. The carrier tube is not waterproof.
- The blue safety release helps to prevent accidental injection. Keep the blue safety release on until you need to use epinephrine injection, USP auto-injector.
-
Your epinephrine injection, USP auto-injector has an expiration date. Replace it before the expiration date. Throw away (dispose of) expired, unwanted, or unused epinephrine injection, USP auto-injectors in an FDA-cleared sharps disposal container. Do not throw away the epinephrine injection, USP auto-injectors in your household trash. If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- o Made of heavy-duty plastic
- o Can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- o Upright and stable during use
- o Leak-resistant, and
- o Properly labeled to warn of hazardous waste inside the container
- Visit the FDA website (www.fda.gov/drugs/safe-disposal-medicines/disposal-unused-medicines-what-you-should-know) for more information about how to throw away (dispose of) unused medicines.
Keep epinephrine injection, USP auto-injectors and all medicines out of the reach of children.
General information about the safe and effective use of epinephrine injection, USP auto-injectors
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not use the epinephrine injection, USP auto-injector for a condition for which it was not prescribed. Do not give your epinephrine injection, USP auto-injector to other people.
This Patient Information Leaflet summarizes the most important information about epinephrine injection, USP auto-injectors. If you would like more information, talk to your healthcare provider. You can ask your pharmacist or healthcare provider for information about epinephrine injection, USP auto-injectors that is written for health professionals.
For more information and video instructions on the use of epinephrine injection, USP auto-injectors, go to MyGenericEAI.com or call 1-800-395-3376.
What are the ingredients in epinephrine injection, USP auto-injectors?
- Active Ingredients: Epinephrine
- Inactive Ingredients: sodium chloride, sodium metabisulfite, hydrochloric acid, and water
Important Information
- The epinephrine injection, USP auto-injector, 0.3 mg has a yellow colored label.
- The epinephrine injection, USP auto-injector, 0.15 mg has a green colored label.
- The epinephrine injection, USP auto-injector Trainer has a grey color and contains no medicine and no needle.
- Your auto-injector is designed to work through clothing.
- The blue safety release on the epinephrine injection, USP auto-injector helps to prevent accidental injection of the device. Do not remove the blue safety release until you are ready to use it.
- Choking hazard: The blue safety release is a small part that may become a choking hazard for children. Throw away the blue safety release immediately after using epinephrine injection, USP auto-injector.
- Only inject into the middle of the outer thigh (upper leg). Never inject into any other part of the body.
- Never put your thumb, fingers, or your hand over the orange tip. The needle comes out of the orange tip.
- If an accidental injection happens, get emergency medical help right away.
-
Do not place patient information or any other foreign objects in the carrier tube with the auto-injector, as this may prevent you from removing the auto-injector for use.
Instructions for Use
Epinephrine Injection, USP Auto-Injector 0.3 mg
one dose of 0.3 mg epinephrine, USP 0.3 mg/0.3 mLEpinephrine Injection, USP Auto-Injector 0.15 mg
one dose of 0.15 mg epinephrine, USP 0.15 mg/0.3 mLFor allergic emergencies (anaphylaxis)
Read this Instructions for Use carefully before you use epinephrine injection, USP auto-injector. Before you need to use your epinephrine injection, USP auto-injector, make sure your healthcare provider shows you the right way to use it. Parents, caregivers, and others who may be in a position to administer epinephrine injection, USP auto-injector should also understand how to use it as well. If you have any questions, ask your healthcare provider.
Your epinephrine injection, USP auto-injectors
A dose of epinephrine injection, USP auto-injector requires 3 steps: Prepare, Administer and Get emergency medical help
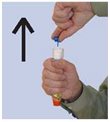
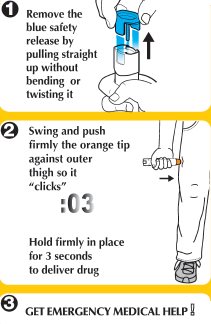
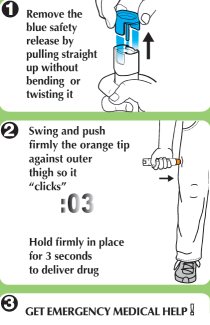
- Step 1. Prepare epinephrine injection, USP auto-injector for injection
- Step 2. Administer epinephrine injection, USP auto-injector
If you are administering epinephrine injection, USP auto-injector to a young child, hold the leg firmly in place while administering an injection.
- Step 3. Get emergency medical help now.
You may need further medical attention. You may need to use a second epinephrine injection, USP auto-injector if symptoms continue or recur.
- Take your used auto-injector with you when you go to see a healthcare provider.
- Tell the healthcare provider that you have received an injection of epinephrine. Show the healthcare provider where you received the injection.
- Give your used epinephrine injection, USP auto-injector to the healthcare provider for inspection and proper disposal.
- Ask for a refill, if needed.
Note:
- The used auto-injector with extended needle cover will not fit in the carrier tube.
- Epinephrine injection, USP auto-injectors are single-use injectable devices that deliver a fixed dose of epinephrine. The auto-injector cannot be reused. Do not attempt to reuse epinephrine injection, USP auto-injector after the device has been activated. It is normal for most of the medicine to remain in the auto-injector after the dose is injected. The correct dose has been administered if the orange needle tip is extended and the window is blocked.
- Your epinephrine injection, USP auto-injectors may come packaged with an epinephrine injection, USP auto-injector Trainer and separate Trainer Instructions for Use. The epinephrine injection, USP auto-injector Trainer has a grey color. The grey epinephrine injection, USP auto-injector Trainer contains no medicine and no needle. Practice with your epinephrine injection, USP auto-injector Trainer, but always carry your real epinephrine injection, USP auto-injector in case of an allergic emergency.
- If you will be administering epinephrine injection, USP auto-injector to a young child, ask your healthcare provider to show you how to properly hold the leg in place while administering a dose.
- Do not try to take the epinephrine injection, USP auto-injector apart.
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
Manufactured for:
Mylan Specialty L.P., Morgantown, WV 26505, U.S.A. by Meridian Medical Technologies, Inc., Columbia, MD 21046, U.S.A., a Pfizer companyCopyright © 2020 Meridian Medical Technologies. All rights reserved.
Revised: 1/2020
MS:PIL:EPIG:R3
0002075Epinephrine Injection, USP Auto-Injector 0.3 mg
one dose of 0.3 mg epinephrine, USP 0.3 mg/0.3 mLEpinephrine Injection, USP Auto-Injector 0.15 mg
one dose of 0.15 mg epinephrine, USP 0.15 mg/0.3 mLMyGenericEAI.com
Register your epinephrine injection, USP auto-injector at MyGenericEAI.com and find out more about:
- Free epinephrine injection, USP auto-injector Refill Reminder Program. It is important to keep your auto-injector up-to-date.
Register up to 6 epinephrine injection, USP auto-injectors and receive automatic Refill Reminder Alerts.
- Receive periodic information related to allergies and allergens.
- Instructional Video
For more information about epinephrine injection, USP auto-injectors and proper use of the product, call Mylan at 1-877-446-3679 or visit MyGenericEAI.com.
Epinephrine Injection, USP Auto-Injector Trainer
Instructions for UseEpinephrine Injection, USP Auto-Injector Trainer Instructions for Use
In an emergency: Do not use the grey Trainer. Use your real yellow epinephrine injection, USP auto-injector, 0.3 mg or green epinephrine injection, USP auto-injector, 0.15 mg.
Important Information
- The Trainer label has a grey color.
- The Trainer contains no medicine and no needle.
- Periodically practice with the grey colored Trainer before an allergic emergency (anaphylaxis) happens to make sure you are able to safely use the real yellow epinephrine injection, USP auto-injector, 0.3 mg or green epinephrine injection, USP auto-injector, 0.15 mg in case of an emergency.
- Always carry your real yellow epinephrine injection, USP auto-injector, 0.3 mg or green epinephrine injection, USP auto-injector, 0.15 mg in case of an allergic emergency.
- Small parts like the blue safety release may become a choking hazard for children. Put the blue safety release back on the Trainer and reset it immediately after practicing.
The Epinephrine Injection, USP Auto-Injector Trainer
Familiarize yourself with this grey Trainer. Practice until you are comfortable using it.
Your grey colored Trainer:
- Never put your thumb, other fingers, or hand over the Orange Tip.
- The Orange Tip is where the needle comes out of your epinephrine injection, USP auto-injector.
Practice Instructions
Note: With the real yellow epinephrine injection, USP auto-injector, 0.3 mg or green epinephrine injection, USP auto-injector, 0.15 mg, the orange tip covers the needle after self-injection to help protect you from accidentally sticking yourself or others.
Practice Session Information
In case of an allergic emergency, use the real yellow epinephrine injection, USP auto-injector, 0.3 mg or green epinephrine injection, USP auto-injector, 0.15 mg and not the grey Trainer.
Follow instructions above. Repeat as often as needed until you are able to self-inject quickly and correctly.
Reread:
- The Trainer Instructions for Use
- The “Patient Information” that comes with your epinephrine injection, USP auto-injector
Train others who could help you in an emergency:
-
Your parents, caregivers, and others who may be in a position to administer epinephrine injection, USP auto-injector should know how to help you during an allergic emergency (anaphylaxis). Before an emergency occurs, have them:
- Practice activating the Trainer
- Read these Trainer Instructions and the “Patient Information”
For more information about Epinephrine Injection, USP Auto-Injectors and the proper use of the products, go to MyGenericEAI.com.
Caution:
Important differences between the Trainer and your real yellow epinephrine injection, USP auto-injector, 0.3 mg or green epinephrine injection, USP auto-injector, 0.15 mg
This Trainer Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for Mylan Specialty L.P., Morgantown, WV 26505, U.S.A. by Meridian Medical Technologies, Inc., Columbia, MD 21046, U.S.A., a Pfizer company
Copyright © 2020 Meridian Medical Technologies. All rights reserved.
Revised: 1/2020
MS:EPITG:R3
0002079 - 1.
Epinephrine injection, USP auto-injector contains epinephrine, a medicine used to treat allergic emergencies (anaphylaxis). Anaphylaxis can be life threatening, can happen within minutes, and can be caused by stinging and biting insects, allergy injections, foods, medicines, exercise, or unknown causes.
-
PRINCIPAL DISPLAY PANEL – 0.3 mg
NDC: 49502-102-02
Rx onlyFor Allergic Emergencies (Anaphylaxis)
Epinephrine Injection, USP
Auto-Injectors 0.3 mgEach carton contains:
Authorized generic for
EpiPen® Auto-InjectorFor more information about your Epinephrine Injection, USP
Auto-Injectors visit MyGenericEAI.comSee enclosed Patient Information Leaflet
for complete instructions for use.Note: The needle comes out of the orange tip.
Never put your thumb, fingers or hand
over the orange tip.Never put thumb, fingers or hand over orange tip.
Do not remove blue safety release until ready to use.Contains 2 Epinephrine Injection, USP Auto-Injectors and 1 Trainer.
Each Epinephrine Injection, USP Auto-Injector delivers one 0.3 mg
intramuscular dose of epinephrine from epinephrine injection, USP
0.3 mg/0.3 mL.Discard unit after use. The Trainer contains no medicine and no needle.
Read enclosed patient package insert carefully before using.Replace if discolored. Store at 68° to 77°F
(20° to 25°C). Do not refrigerate.
Protect from light.Manufactured for Mylan Specialty L.P., Morgantown, WV 26505 USA
by Meridian Medical Technologies, Inc.,
Columbia, MD 21046, USA, a Pfizer company.Copyright© 2020 Meridian Medical Technologies. All rights reserved.
0002077
MS:10202:2C:R4 -
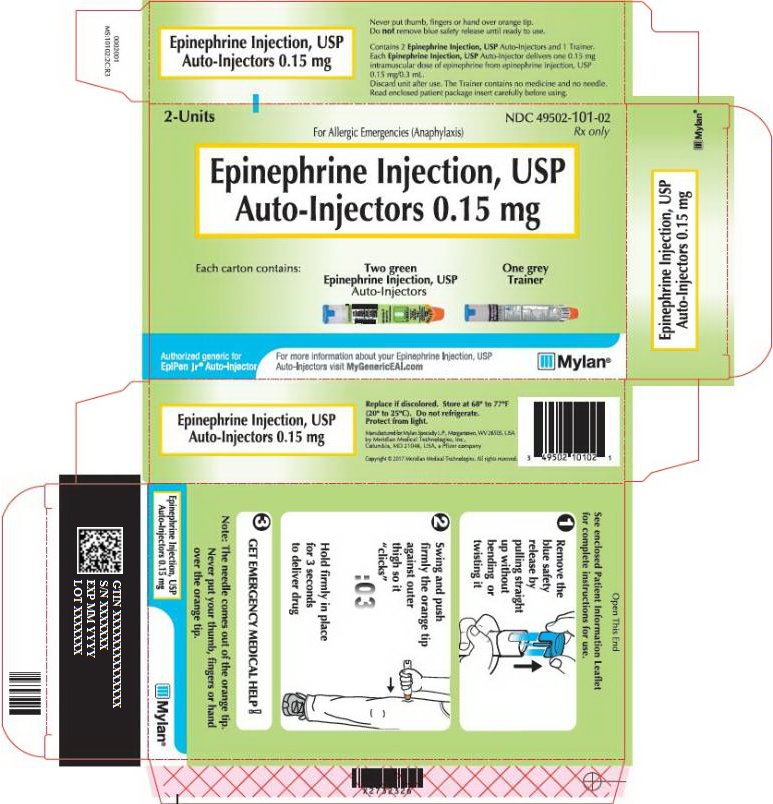
PRINCIPAL DISPLAY PANEL – 0.15 mg
NDC: 49502-101-02
Rx onlyFor Allergic Emergencies (Anaphylaxis)
Epinephrine Injection, USP
Auto-Injectors 0.15 mgEach carton contains:
Authorized generic for
EpiPen Jr® Auto-InjectorFor more information about your Epinephrine Injection, USP
Auto-Injectors visit MyGenericEAI.comSee enclosed Patient Information Leaflet
for complete instructions for use.Note: The needle comes out of the orange tip.
Never put your thumb, fingers or hand
over the orange tip.Never put thumb, fingers or hand over orange tip.
Do not remove blue safety release until ready to use.Contains 2 Epinephrine Injection, USP Auto-Injectors and 1 Trainer.
Each Epinephrine Injection, USP Auto-Injector delivers one 0.15 mg
intramuscular dose of epinephrine from epinephrine injection, USP
0.15 mg/0.3 mL.Discard unit after use. The Trainer contains no medicine and no needle.
Read enclosed patient package insert carefully before using.Replace if discolored. Store at 68° to 77°F
(20° to 25°C). Do not refrigerate.
Protect from light.Manufactured for Mylan Specialty L.P., Morgantown, WV 26505 USA
by Meridian Medical Technologies, Inc.,
Columbia, MD 21046, USA, a Pfizer company.Copyright© 2020 Meridian Medical Technologies. All rights reserved.
0002078
MS:10102:2C:R4 -
INGREDIENTS AND APPEARANCE
EPINEPHRINE
epinephrine injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 49502-102 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE (UNII: YKH834O4BH) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.3 mg in 0.3 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM METABISULFITE (UNII: 4VON5FNS3C) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49502-102-02 2 in 1 CARTON 12/15/2016 1 NDC: 49502-102-01 1 in 1 CONTAINER 1 0.3 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA019430 12/15/2016 EPINEPHRINE
epinephrine injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 49502-101 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE (UNII: YKH834O4BH) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.15 mg in 0.3 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM METABISULFITE (UNII: 4VON5FNS3C) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49502-101-02 2 in 1 CARTON 12/15/2016 1 NDC: 49502-101-01 1 in 1 CONTAINER 1 0.3 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA019430 12/15/2016 Labeler - Mylan Specialty L.P. (194775557)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.