COSENTYX- secukinumab injection
COSENTYX by
Drug Labeling and Warnings
COSENTYX by is a Prescription medication manufactured, distributed, or labeled by Novartis Pharmaceuticals Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use COSENTYX safely and effectively. See full prescribing information for COSENTYX.
COSENTYX® (secukinumab) injection, for subcutaneous use
COSENTYX® (secukinumab) for injection, for subcutaneous use
Initial U.S. Approval: 2015RECENT MAJOR CHANGES
Dosage and Administration (2.3)
1/2020
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
Plaque Psoriasis
- Recommended dosage is 300 mg by subcutaneous injection at Weeks 0, 1, 2, 3, and 4 followed by 300 mg every 4 weeks. For some patients, a dose of 150 mg may be acceptable. (2.1)
Psoriatic Arthritis
- For psoriatic arthritis patients with coexistent moderate to severe plaque psoriasis, use the dosage and administration for plaque psoriasis. (2.1)
- For other psoriatic arthritis patients administer with or without a loading dosage. The recommended dosage:
- With a loading dosage is 150 mg at weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter
- Without a loading dosage is 150 mg every 4 weeks
- If a patient continues to have active psoriatic arthritis, consider a dosage of 300 mg every 4 weeks. (2.2)
Ankylosing Spondylitis
- Administer with or without a loading dosage. The recommended dosage:
- With a loading dosage is 150 mg at weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter
- Without a loading dosage is 150 mg every 4 weeks
- If a patient continues to have active ankylosing spondylitis, consider a dosage of 300 mg every 4 weeks (2.3)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
Serious hypersensitivity reaction to secukinumab or to any of the excipients. (4)
WARNINGS AND PRECAUTIONS
- Infections: Serious infections have occurred. Caution should be exercised when considering the use of COSENTYX in patients with a chronic infection or a history of recurrent infection. If a serious infection develops, discontinue COSENTYX until the infection resolves. (5.1)
- Tuberculosis (TB): Prior to initiating treatment with COSENTYX, evaluate for TB. (5.2)
- Inflammatory Bowel Disease: Cases of inflammatory bowel disease were observed in clinical trials. Caution should be exercised when prescribing COSENTYX to patients with inflammatory bowel disease. (5.3)
- Hypersensitivity Reactions: If an anaphylactic reaction or other serious allergic reaction occurs, discontinue COSENTYX immediately and initiate appropriate therapy. (5.4)
ADVERSE REACTIONS
Most common adverse reactions (greater than 1%) are nasopharyngitis, diarrhea, and upper respiratory tract infection. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 1/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Plaque Psoriasis
1.2 Psoriatic Arthritis
1.3 Ankylosing Spondylitis
2 DOSAGE AND ADMINISTRATION
2.1 Plaque Psoriasis
2.2 Psoriatic Arthritis
2.3 Ankylosing Spondylitis
2.4 Assessment Prior to Initiation of COSENTYX
2.5 Important Administration Instructions
2.6 Preparation for Use of COSENTYX Sensoready® Pen and Prefilled Syringe
2.7 Reconstitution and Preparation of COSENTYX Lyophilized Powder
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Infections
5.2 Pre-treatment Evaluation for Tuberculosis
5.3 Inflammatory Bowel Disease
5.4 Hypersensitivity Reactions
5.5 Risk of Hypersensitivity in Latex-sensitive Individuals
5.6 Vaccinations
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
7 DRUG INTERACTIONS
7.1 Live Vaccines
7.2 Non-Live Vaccines
7.3 CYP450 Substrates
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Plaque Psoriasis
14.2 Psoriatic Arthritis
14.3 Ankylosing Spondylitis
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Plaque Psoriasis
The recommended dosage is 300 mg by subcutaneous injection at Weeks 0, 1, 2, 3, and 4 followed by 300 mg every 4 weeks. Each 300 mg dosage is given as 2 subcutaneous injections of 150 mg.
For some patients, a dosage of 150 mg may be acceptable.
2.2 Psoriatic Arthritis
For psoriatic arthritis patients with coexistent moderate to severe plaque psoriasis, use the dosing and administration recommendations for plaque psoriasis [see Dosage and Administration (2.1)].
For other psoriatic arthritis patients, administer COSENTYX with or without a loading dosage by subcutaneous injection. The recommended dosage:
- With a loading dosage is 150 mg at weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter
- Without a loading dosage is 150 mg every 4 weeks
- If a patient continues to have active psoriatic arthritis, consider a dosage of 300 mg every 4 weeks.
COSENTYX may be administered with or without methotrexate.
2.3 Ankylosing Spondylitis
Administer COSENTYX with or without a loading dosage by subcutaneous injection. The recommended dosage:
- With a loading dosage is 150 mg at weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter
- Without a loading dosage is 150 mg every 4 weeks
- If a patient continues to have active ankylosing spondylitis, consider a dosage of 300 mg every 4 weeks.
2.4 Assessment Prior to Initiation of COSENTYX
Evaluate patients for tuberculosis (TB) infection prior to initiating treatment with COSENTYX [see Warnings and Precautions (5.2)].
2.5 Important Administration Instructions
There are three presentations for COSENTYX (i.e., Sensoready pen, prefilled syringe, and lyophilized powder in vial for reconstitution). The COSENTYX “Instructions for Use” for each presentation contains more detailed instructions on the preparation and administration of COSENTYX [see Instructions for Use].
COSENTYX is intended for use under the guidance and supervision of a physician. Patients may self-inject after proper training in subcutaneous injection technique using the Sensoready pen or prefilled syringe and when deemed appropriate. The lyophilized powder for reconstitution is for healthcare provider use only. Administer each injection at a different anatomic location (such as upper arms, thighs, or any quadrant of abdomen) than the previous injection, and not into areas where the skin is tender, bruised, erythematous, indurated, or affected by psoriasis. Administration of COSENTYX in the upper, outer arm may be performed by a caregiver or healthcare provider.
2.6 Preparation for Use of COSENTYX Sensoready® Pen and Prefilled Syringe
Before injection, remove COSENTYX Sensoready pen or COSENTYX prefilled syringe from the refrigerator and allow COSENTYX to reach room temperature (15 to 30 minutes) without removing the needle cap.
The removable cap of the COSENTYX Sensoready pen and the COSENTYX prefilled syringe contains natural rubber latex and should not be handled by latex-sensitive individuals [see Warnings and Precautions (5.5)].
Inspect COSENTYX visually for particulate matter and discoloration prior to administration. COSENTYX injection is a clear to slightly opalescent, colorless to slightly yellow solution. Do not use if the liquid contains visible particles, is discolored or cloudy. COSENTYX does not contain preservatives; therefore, administer the Sensoready pen or prefilled syringe within 1 hour after removal from the refrigerator. Discard any unused product remaining in the Sensoready pen or prefilled syringe.
2.7 Reconstitution and Preparation of COSENTYX Lyophilized Powder
COSENTYX lyophilized powder should be prepared and reconstituted with Sterile Water for Injection by a trained healthcare provider using aseptic technique and without interruption. The preparation time from piercing the stopper until end of reconstitution on average takes 20 minutes and should not exceed 90 minutes.
a) Remove the vial of COSENTYX lyophilized powder from the refrigerator and allow to stand for 15 to 30 minutes to reach room temperature. Ensure the Sterile Water for Injection is at room temperature.
b) Slowly inject 1 mL of Sterile Water for Injection into the vial containing COSENTYX lyophilized powder and direct the stream of Sterile Water for Injection onto the lyophilized powder.
c) Tilt the vial at an angle of approximately 45 degrees and gently rotate between the fingertips for approximately 1 minute. Do not shake or invert the vial.
d) Allow the vial to stand for about 10 minutes at room temperature to allow for dissolution. Note that foaming may occur.
e) Tilt the vial at an angle of approximately 45 degrees and gently rotate between the fingertips for approximately 1 minute. Do not shake or invert the vial.
f) Allow the vial to stand undisturbed at room temperature for approximately 5 minutes. The reconstituted COSENTYX solution should be essentially free of visible particles, clear to opalescent, and colorless to slightly yellow. Do not use if the lyophilized powder has not fully dissolved or if the liquid contains visible particles, is cloudy or discolored.
g) Prepare the required number of vials (1 vial for the 150 mg dose or 2 vials for the 300 mg dose).
h) The COSENTYX reconstituted solution contains 150 mg of secukinumab in 1 mL of solution. After reconstitution, use the solution immediately or store in the refrigerator at 2ºC to 8ºC (36ºF to 46ºF) for up to 24 hours. Do not freeze.
i) If stored at 2ºC to 8ºC (36ºF to 46ºF), allow the reconstituted COSENTYX solution to reach room temperature (15 to 30 minutes) before administration. COSENTYX does not contain preservatives; therefore, administer within 1 hour after removal from 2ºC to 8ºC (36ºF to 46ºF) storage.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Infections
COSENTYX may increase the risk of infections. In clinical trials, a higher rate of infections was observed in COSENTYX treated subjects compared to placebo-treated subjects. In placebo-controlled clinical trials in patients with moderate to severe plaque psoriasis, higher rates of common infections such as nasopharyngitis (11.4% versus 8.6%), upper respiratory tract infection (2.5% versus 0.7%) and mucocutaneous infections with candida (1.2% versus 0.3%) were observed with COSENTYX compared with placebo. A similar increase in risk of infection was seen in placebo-controlled trials in patients with psoriatic arthritis and ankylosing spondylitis [see Adverse Reactions (6.1)]. The incidence of some types of infections appeared to be dose-dependent in clinical studies [see Adverse Reactions (6.1)].
Exercise caution when considering the use of COSENTYX in patients with a chronic infection or a history of recurrent infection.
Instruct patients to seek medical advice if signs or symptoms suggestive of an infection occur. If a patient develops a serious infection, the patient should be closely monitored and COSENTYX should be discontinued until the infection resolves.
5.2 Pre-treatment Evaluation for Tuberculosis
Evaluate patients for tuberculosis (TB) infection prior to initiating treatment with COSENTYX. Do not administer COSENTYX to patients with active TB infection. Initiate treatment of latent TB prior to administering COSENTYX. Consider anti-TB therapy prior to initiation of COSENTYX in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed. Patients receiving COSENTYX should be monitored closely for signs and symptoms of active TB during and after treatment.
5.3 Inflammatory Bowel Disease
Caution should be used when prescribing COSENTYX to patients with inflammatory bowel disease. Exacerbations, in some cases serious, occurred in COSENTYX treated patients during clinical trials in plaque psoriasis, psoriatic arthritis and ankylosing spondylitis. In addition, new onset inflammatory bowel disease cases occurred in clinical trials with COSENTYX. In an exploratory study in 59 patients with active Crohn’s disease, there were trends toward greater disease activity and increased adverse events in the secukinumab group as compared to the placebo group. Patients who are treated with COSENTYX should be monitored for signs and symptoms of inflammatory bowel disease [see Adverse Reactions (6.1)].
5.4 Hypersensitivity Reactions
Anaphylaxis and cases of urticaria occurred in COSENTYX treated patients in clinical trials. If an anaphylactic or other serious allergic reaction occurs, administration of COSENTYX should be discontinued immediately and appropriate therapy initiated [see Adverse Reactions (6.1)].
5.5 Risk of Hypersensitivity in Latex-sensitive Individuals
The removable cap of the COSENTYX Sensoready pen and the COSENTYX prefilled syringe contains natural rubber latex which may cause an allergic reaction in latex-sensitive individuals. The safe use of COSENTYX Sensoready pen or prefilled syringe in latex-sensitive individuals has not been studied.
5.6 Vaccinations
Prior to initiating therapy with COSENTYX, consider completion of all age appropriate immunizations according to current immunization guidelines. Patients treated with COSENTYX should not receive live vaccines.
Non-live vaccinations received during a course of COSENTYX may not elicit an immune response sufficient to prevent disease.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail elsewhere in the labeling:
- Infections [see Warnings and Precautions (5.1)]
- Inflammatory Bowel Disease [see Warnings and Precautions (5.3)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Plaque Psoriasis
A total of 3430 plaque psoriasis subjects were treated with COSENTYX in controlled and uncontrolled clinical trials. Of these, 1641 subjects were exposed for at least 1 year.
Four placebo-controlled phase 3 trials in plaque psoriasis subjects were pooled to evaluate the safety of COSENTYX in comparison to placebo up to 12 weeks after treatment initiation, in Trials 1, 2, 3, and 4. In total, 2077 subjects were evaluated (691 to COSENTYX 300 mg group, 692 to COSENTYX 150 mg group, and 694 to placebo group) [see Clinical Studies (14)].
Table 1 summarizes the adverse reactions that occurred at a rate of at least 1% and at a higher rate in the COSENTYX groups than the placebo group during the 12-week placebo-controlled period of the placebo-controlled trials.
Table 1: Adverse Reactions Reported by Greater Than 1% of Subjects with Plaque Psoriasis Through Week 12 in Trials 1, 2, 3, and 4 COSENTYX Adverse Reactions 300 mg
(N = 691)
n (%)150 mg
(N = 692)
n (%)Placebo
(N = 694)
n (%)Nasopharyngitis 79 (11.4) 85 (12.3) 60 (8.6) Diarrhea 28 (4.1) 18 (2.6) 10 (1.4) Upper respiratory tract infection 17 (2.5) 22 (3.2) 5 (0.7) Rhinitis 10 (1.4) 10 (1.4) 5 (0.7) Oral herpes 9 (1.3) 1 (0.1) 2 (0.3) Pharyngitis 8 (1.2) 7 (1.0) 0 (0) Urticaria 4 (0.6) 8 (1.2) 1 (0.1) Rhinorrhea 8 (1.2) 2 (0.3) 1 (0.1) Adverse reactions that occurred at rates less than 1% in the placebo-controlled period of Trials 1, 2, 3, and 4 through Week 12 included: sinusitis, tinea pedis, conjunctivitis, tonsillitis, oral candidiasis, impetigo, otitis media, otitis externa, inflammatory bowel disease, increased liver transaminases, and neutropenia.
Infections
In the placebo-controlled period of the clinical trials in plaque psoriasis (a total of 1382 subjects treated with COSENTYX and 694 subjects treated with placebo up to 12 weeks), infections were reported in 28.7% of subjects treated with COSENTYX compared with 18.9% of subjects treated with placebo. Serious infections occurred in 0.14% of patients treated with COSENTYX and in 0.3% of patients treated with placebo [see Warnings and Precautions (5.1)].
Over the entire treatment period (a total of 3430 plaque psoriasis subjects treated with COSENTYX for up to 52 weeks for the majority of subjects), infections were reported in 47.5% of subjects treated with COSENTYX (0.9 per patient-year of follow-up). Serious infections were reported in 1.2% of subjects treated with COSENTYX (0.015 per patient-year of follow-up).
Phase 3 data showed an increasing trend for some types of infection with increasing serum concentration of secukinumab. Candida infections, herpes viral infections, staphylococcal skin infections, and infections requiring treatment increased as serum concentration of secukinumab increased.
Neutropenia was observed in clinical trials. Most cases of secukinumab-associated neutropenia were transient and reversible. No serious infections were associated with cases of neutropenia.
Inflammatory Bowel Disease
Cases of inflammatory bowel disease, in some cases serious, were observed in clinical trials with COSENTYX. In the plaque psoriasis program, with 3430 patients exposed to COSENTYX over the entire treatment period for up to 52 weeks (2725 patient-years), there were 3 cases (0.11 per 100 patient-years) of exacerbation of Crohn’s disease, 2 cases (0.08 per 100 patient-years) of exacerbation of ulcerative colitis, and 2 cases (0.08 per 100 patient-years) of new onset ulcerative colitis. There were no cases in placebo patients (N = 793; 176 patient-years) during the 12 week placebo-controlled period.
One case of exacerbation of Crohn’s disease was reported from long-term non-controlled portions of ongoing clinical trials in plaque psoriasis [see Warnings and Precautions (5.3)].
Hypersensitivity Reactions
Anaphylaxis and cases of urticaria occurred in COSENTYX treated patients in clinical trials [see Warnings and Precautions (5.4)].
Psoriatic Arthritis
COSENTYX was studied in two placebo-controlled psoriatic arthritis trials with 1003 patients (703 patients on COSENTYX and 300 patients on placebo). Of the 703 patients who received COSENTYX, 299 patients received a subcutaneous loading dose of COSENTYX (PsA1) and 404 patients received an intravenous loading dose of secukinumab (PsA2) followed by COSENTYX administered by subcutaneous injection every four weeks. During the 16-week placebo-controlled period of the trials in patients with psoriatic arthritis, the overall proportion of patients with adverse events was similar in the secukinumab and placebo-treatment groups (59% and 58%, respectively). The adverse events that occurred at a proportion of at least 2% and at a higher proportion in the COSENTYX groups than the placebo groups during the 16-week placebo-controlled period were nasopharyngitis, upper respiratory tract infection, headache, nausea, and hypercholesterolemia. The safety profile observed in patients with psoriatic arthritis treated with COSENTYX is consistent with the safety profile in psoriasis.
Similar to the clinical trials in patients with psoriasis, there was an increased proportion of patients with infections in the COSENTYX groups (29%) compared to placebo group (26%) [see Warnings and Precautions (5.1)].
There were cases of Crohn’s disease and ulcerative colitis that include patients who experienced either exacerbations or the development of new disease. There were three cases of inflammatory bowel disease, of which two patients received secukinumab and one received placebo [see Warnings and Precautions (5.3)].
Ankylosing Spondylitis
COSENTYX was studied in two placebo controlled ankylosing spondylitis trials with 590 patients (394 patients on COSENTYX and 196 patients on placebo). Of the 394 patients who received COSENTYX, 145 patients received a subcutaneous load of COSENTYX (study AS1), and 249 received an intravenous loading dose of secukinumab (study AS2) followed by COSENTYX administered by subcutaneous injection every four weeks. During the 16-week placebo-controlled period of the trials in patients with ankylosing spondylitis, the overall proportion of patients with adverse events was higher in the secukinumab groups than the placebo-treatment groups (66% and 59%, respectively). The adverse events that occurred at a proportion of at least 2% and at a higher proportion in the COSENTYX groups than the placebo groups during the 16-week placebo-controlled period were nasopharyngitis, nausea, and upper respiratory tract infection. The safety profile observed in patients with ankylosing spondylitis treated with COSENTYX is consistent with the safety profile in psoriasis. In a third controlled study of AS (study AS3), the safety profile of the 300 mg dose of COSENTYX was consistent with the safety profile of the 150 mg dose of COSENTYX.
Similar to clinical trials in patients with psoriasis, there was an increased proportion of patients with infections in the COSENTYX groups (31%) compared to the placebo group (18%) [see Warnings and Precautions (5.1)].
In the original ankylosing spondylitis program, with 571 patients exposed to COSENTYX there were 8 cases of inflammatory bowel disease during the entire treatment period [5 Crohn’s (0.7 per 100 patient-years) and 3 ulcerative colitis (0.4 per 100 patient-years)]. During the placebo-controlled 16-week period, there were 2 Crohn’s disease exacerbations and 1 new onset ulcerative colitis case that was a serious adverse event in patients treated with COSENTYX compared to none of the patients treated with placebo. During the remainder of the study when all patients received COSENTYX, 1 patient developed Crohn’s disease, 2 patients had Crohn’s exacerbations, 1 patient developed ulcerative colitis, and 1 patient had an ulcerative colitis exacerbation [see Warnings and Precautions (5.3)].
6.2 Immunogenicity
As with all therapeutic proteins, there is the potential for immunogenicity. The immunogenicity of COSENTYX was evaluated using an electrochemiluminescence-based bridging immunoassay. Less than 1% of subjects treated with COSENTYX developed antibodies to secukinumab in up to 52 weeks of treatment. However, this assay has limitations in detecting anti-secukinumab antibodies in the presence of secukinumab; therefore the incidence of antibody development might not have been reliably determined. Of the subjects who developed antidrug antibodies, approximately one-half had antibodies that were classified as neutralizing. Neutralizing antibodies were not associated with loss of efficacy.
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of incidence of antibodies to COSENTYX with the incidences of antibodies to other products may be misleading.
-
7 DRUG INTERACTIONS
7.1 Live Vaccines
Patients treated with COSENTYX may not receive live vaccinations [see Warnings and Precautions (5.6)].
7.2 Non-Live Vaccines
Patients treated with COSENTYX may receive non-live vaccinations. Healthy individuals who received a single 150 mg dose of COSENTYX 2 weeks prior to vaccination with a non-U.S. approved group C meningococcal polysaccharide conjugate vaccine and a non-U.S. approved inactivated seasonal influenza vaccine had similar antibody responses compared to individuals who did not receive COSENTYX prior to vaccination. The clinical effectiveness of meningococcal and influenza vaccines has not been assessed in patients undergoing treatment with COSENTYX [see Warnings and Precautions (5.6)].
7.3 CYP450 Substrates
The formation of CYP450 enzymes can be altered by increased levels of certain cytokines (e.g., IL-1, IL-6, IL-10, TNFα, IFN) during chronic inflammation.
Results from a drug-drug interaction study in subjects with moderate to severe psoriasis showed no clinically relevant interaction for drugs metabolized by CYP3A4.
Upon initiation or discontinuation of COSENTYX in patients who are receiving concomitant CYP450 substrates, particularly those with a narrow therapeutic index, consider monitoring for therapeutic effect or drug concentration and consider dosage adjustment as needed [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited available human data with COSENTYX use in pregnant women are insufficient to inform a drug associated risk of adverse developmental outcomes. In an embryo-fetal development study, no adverse developmental effects were observed in infants born to pregnant monkeys after subcutaneous administration of secukinumab during organogenesis at doses up to 30 times the maximum recommended human dose (MRHD) (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown; however, the background risk in the U.S. general population of major birth defects is 2%-4% and of miscarriage is 15%-20% of clinically recognized pregnancies.
Data
Animal Data
An embryo-fetal development study was performed in cynomolgus monkeys with secukinumab. No malformations or embryo-fetal toxicity were observed in fetuses from pregnant monkeys that were administered secukinumab weekly by the subcutaneous route during the period of organogenesis at doses up to 30 times the MRHD (on a mg/kg basis at a maternal dose of 150 mg/kg).
A pre- and post-natal development toxicity study was performed in mice with a murine analog of secukinumab. No treatment related effects on functional, morphological or immunological development were observed in fetuses from pregnant mice that were administered the murine analog of secukinumab on gestation days 6, 11, and 17 and on postpartum days 4, 10, and 16 at doses up to 150 mg/kg/dose.
8.2 Lactation
Risk Summary
It is not known whether secukinumab is excreted in human milk or absorbed systemically after ingestion. There are no data on the effects of COSENTYX on the breastfed child or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for COSENTYX and any potential adverse effects on the breastfed child from COSENTYX or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of COSENTYX in pediatric patients have not been evaluated.
8.5 Geriatric Use
Of the 3430 plaque psoriasis subjects exposed to COSENTYX in clinical trials, a total of 230 were 65 years or older, and 32 subjects were 75 years or older. Although no differences in safety or efficacy were observed between older and younger subjects, the number of subjects aged 65 years and older was not sufficient to determine whether they responded differently from younger subjects.
- 10 OVERDOSAGE
-
11 DESCRIPTION
Secukinumab is a recombinant human monoclonal IgG1/κ antibody that binds specifically to IL-17A. It is expressed in a recombinant Chinese Hamster Ovary (CHO) cell line. Secukinumab has a molecular mass of approximately 151 kDa; both heavy chains of secukinumab contain oligosaccharide chains.
COSENTYX Injection
COSENTYX injection is a sterile, preservative-free, clear to slightly opalescent, colorless to slightly yellow solution. COSENTYX is supplied in a single-use Sensoready pen with a 27-gauge fixed ½-inch needle, or a single-use prefilled syringe with a 27-gauge fixed ½-inch needle. The removable cap of the COSENTYX Sensoready pen or prefilled syringe contains natural rubber latex.
Each COSENTYX Sensoready pen or prefilled syringe contains 150 mg of secukinumab formulated in: L-histidine/histidine hydrochloride monohydrate (3.103 mg), L-methionine (0.746 mg), polysorbate 80 (0.2 mg), trehalose dihydrate (75.67 mg), and Sterile Water for Injection, USP, at pH of 5.8.
COSENTYX for Injection
COSENTYX for injection is supplied as a sterile, preservative free, white to slightly yellow, lyophilized powder in single-use vials. Each COSENTYX vial contains 150 mg of secukinumab formulated in L-histidine/histidine hydrochloride monohydrate (4.656 mg), polysorbate 80 (0.6 mg), and sucrose (92.43 mg). Following reconstitution with 1 mL Sterile Water for Injection, USP, the resulting pH is approximately 5.8.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Secukinumab is a human IgG1 monoclonal antibody that selectively binds to the interleukin-17A (IL-17A) cytokine and inhibits its interaction with the IL-17 receptor. IL-17A is a naturally occurring cytokine that is involved in normal inflammatory and immune responses. Secukinumab inhibits the release of proinflammatory cytokines and chemokines.
12.2 Pharmacodynamics
Elevated levels of IL-17A are found in psoriatic plaques. Treatment with COSENTYX may reduce epidermal neutrophils and IL-17A levels in psoriatic plaques. Serum levels of total IL-17A (free and secukinumab-bound IL-17A) measured at Week 4 and Week 12 were increased following secukinumab treatment. These pharmacodynamic activities are based on small exploratory studies. The relationship between these pharmacodynamic activities and the mechanism(s) by which secukinumab exerts its clinical effects is unknown.
Increased numbers of IL-17A producing lymphocytes and innate immune cells and increased levels of IL-17A have been found in the blood of patients with psoriatic arthritis and ankylosing spondylitis.
12.3 Pharmacokinetics
The PK properties of secukinumab observed in psoriatic arthritis and ankylosing spondylitis patients were similar to the PK properties displayed in plaque psoriasis patients.
Absorption
Following a single subcutaneous dose of either 150 mg (one-half the recommended dose) or 300 mg in plaque psoriasis patients, secukinumab reached peak mean (± SD) serum concentrations (Cmax) of 13.7 ± 4.8 mcg/mL and 27.3 ± 9.5 mcg/mL, respectively, by approximately 6 days post dose.
Following multiple subcutaneous doses of secukinumab, the mean (± SD) serum trough concentrations of secukinumab ranged from 22.8 ± 10.2 mcg/mL (150 mg) to 45.4 ± 21.2 mcg/mL (300 mg) at Week 12. At the 300 mg dose at Week 4 and Week 12, the mean trough concentrations resulted from the Sensoready pen were 23% to 30% higher than those from the lyophilized powder and 23% to 26% higher than those from the prefilled syringe based on cross-study comparisons.
Steady-state concentrations of secukinumab were achieved by Week 24 following the every 4 week dosing regimens. The mean (± SD) steady-state trough concentrations ranged from 16.7 ± 8.2 mcg/mL (150 mg) to 34.4 ± 16.6 mcg/mL (300 mg).
In healthy subjects and subjects with plaque psoriasis, secukinumab bioavailability ranged from 55% to 77% following subcutaneous dose of 150 mg (one-half the recommended dose) or 300 mg.
Distribution
The mean volume of distribution during the terminal phase (Vz) following a single intravenous administration ranged from 7.10 to 8.60 L in plaque psoriasis patients. Intravenous use is not recommended [see Dosage and Administration (2)].
Secukinumab concentrations in interstitial fluid in lesional and non-lesional skin of plaque psoriasis patients ranged from 27% to 40% of those in serum at 1 and 2 weeks after a single subcutaneous dose of secukinumab 300 mg.
Elimination
The metabolic pathway of secukinumab has not been characterized. As a human IgG1κ monoclonal antibody secukinumab is expected to be degraded into small peptides and amino acids via catabolic pathways in the same manner as endogenous IgG.
The mean systemic clearance (CL) ranged from 0.14 L/day to 0.22 L/day and the mean half-life ranged from 22 to 31 days in plaque psoriasis subjects following intravenous and subcutaneous administration across all psoriasis trials. Intravenous use is not recommended [see Dosage and Administration (2)].
Dose Linearity
Secukinumab exhibited dose-proportional pharmacokinetics in subjects with psoriasis over a dose range from 25 mg (approximately 0.083 times the recommended dose) to 300 mg following subcutaneous administrations.
Weight
Secukinumab clearance and volume of distribution increase as body weight increases.
Specific Populations
Hepatic or Renal Impairment:
No formal trial of the effect of hepatic or renal impairment on the pharmacokinetics of secukinumab was conducted.
Age: Geriatric Population:
Population pharmacokinetic analysis indicated that the clearance of secukinumab was not significantly influenced by age in adult subjects with plaque psoriasis, psoriatic arthritis and ankylosing spondylitis. Subjects who are 65 years or older had apparent clearance of secukinumab similar to subjects less than 65 years old.
Drug Interactions
Cytochrome P450 Substrates
In subjects with plaque psoriasis, midazolam (CYP3A4 substrate) pharmacokinetics was similar when administered alone, or when administered following either a single or five weekly subcutaneous administrations of 300 mg secukinumab [see Drug interactions (7.3)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies have not been conducted to evaluate the carcinogenic or mutagenic potential of COSENTYX. Some published literature suggests that IL-17A directly promotes cancer cell invasion in vitro, whereas other reports indicate IL-17A promotes T-cell mediated tumor rejection. Depletion of IL-17A with a neutralizing antibody inhibited tumor development in mice. The relevance of experimental findings in mouse models for malignancy risk in humans is unknown.
No effects on fertility were observed in male and female mice that were administered a murine analog of secukinumab at subcutaneous doses up to 150 mg/kg once weekly prior to and during the mating period.
-
14 CLINICAL STUDIES
14.1 Plaque Psoriasis
Four multicenter, randomized, double-blind, placebo-controlled trials (Trials 1, 2, 3, and 4) enrolled 2403 subjects (691 randomized to COSENTYX 300 mg, 692 to COSENTYX 150 mg, 694 to placebo, and 323 to a biologic active control) 18 years of age and older with plaque psoriasis who had a minimum body surface area involvement of 10%, and Psoriasis Area and Severity Index (PASI) score greater than or equal to 12, and who were candidates for phototherapy or systemic therapy.
- Trial 1 enrolled 738 subjects (245 randomized to COSENTYX 300 mg, 245 to COSENTYX 150 mg, and 248 to placebo). Subjects received subcutaneous treatment at Weeks 0, 1, 2, 3, and 4 followed by dosing every 4 weeks. Subjects randomized to COSENTYX received 300 mg or 150 mg doses at Weeks 0, 1, 2, 3, and 4 followed by the same dose every 4 weeks. Subjects randomized to receive placebo that were non-responders at Week 12 were then crossed over to receive COSENTYX (either 300 mg or 150 mg) at Weeks 12, 13, 14, 15, and 16 followed by the same dose every 4 weeks. All subjects were followed for up to 52 weeks following first administration of study treatment.
- Trial 2 enrolled 1306 subjects (327 randomized to COSENTYX 300 mg, 327 to COSENTYX 150 mg, 326 to placebo, and 323 to a biologic active control). COSENTYX and placebo data are described. Subjects received subcutaneous treatment at Weeks 0, 1, 2, 3, and 4 followed by dosing every 4 weeks. Subjects randomized to COSENTYX received 300 mg or 150 mg doses at Weeks 0, 1, 2, 3, and 4 followed by the same dose every 4 weeks. Subjects randomized to receive placebo that were non-responders at Week 12 then crossed over to receive COSENTYX (either 300 mg or 150 mg) at Weeks 12, 13, 14, 15, and 16 followed by the same dose every 4 weeks. All subjects were followed for up to 52 weeks following first administration of study treatment.
- Trial 3 enrolled 177 subjects (59 randomized to COSENTYX 300 mg, 59 to COSENTYX 150 mg, and 59 to placebo) and assessed safety, tolerability, and usability of COSENTYX self-administration via prefilled syringe for 12 weeks. Subjects received subcutaneous treatment at Weeks 0, 1, 2, 3, and 4, followed by the same dose every 4 weeks for up to 12 weeks total.
- Trial 4 enrolled 182 subjects (60 randomized to COSENTYX 300 mg, 61 to COSENTYX 150 mg, and 61 to placebo) and assessed safety, tolerability, and usability of COSENTYX self-administration via Sensoready pen for 12 weeks. Subjects received subcutaneous treatment at Weeks 0, 1, 2, 3, and 4, followed by the same dose every 4 weeks for up to 12 weeks total.
Endpoints
In all trials, the endpoints were the proportion of subjects who achieved a reduction in PASI score of at least 75% (PASI 75) from baseline to Week 12 and treatment success (clear or almost clear) on the Investigator’s Global Assessment modified 2011 (IGA). Other evaluated outcomes included the proportion of subjects who achieved a reduction in PASI score of at least 90% (PASI 90) from baseline at Week 12, maintenance of efficacy to Week 52, and improvements in itching, pain, and scaling at Week 12 based on the Psoriasis Symptom Diary©.
The PASI is a composite score that takes into consideration both the percentage of body surface area affected and the nature and severity of psoriatic changes within the affected regions (induration, erythema and scaling). The IGA is a 5-category scale including “0 = clear”, “1 = almost clear”, “2 = mild”, “3 = moderate” or “4 = severe” indicating the physician’s overall assessment of the psoriasis severity focusing on induration, erythema and scaling. Treatment success of “clear” or “almost clear” consisted of no signs of psoriasis or normal to pink coloration of lesions, no thickening of the plaque, and none to minimal focal scaling.
Baseline Characteristics
Across all treatment groups the baseline PASI score ranged from 11 to 72 with a median of 20 and the baseline IGA score ranged from “moderate” (62%) to “severe” (38%). Of the 2077 plaque psoriasis subjects who were included in the placebo-controlled trials, 79% were biologic-naïve (have never received a prior treatment with biologics) and 45% were non-biologic failures (failed to respond to a prior treatment with non-biologics therapies). Of the patients who received a prior treatment with biologics, over one-third were biologic failures. Approximately 15% to 25% of trial subjects had a history of psoriatic arthritis.
Clinical Response
The results of Trials 1 and 2 are presented in Table 2.
Table 2: Clinical Outcomes at Week 12 in Adults with Plaque Psoriasis in Trials 1 and 2 Trial 1 Trial 2 COSENTYX
300 mg
(N = 245)
n (%)COSENTYX
150 mg
(N = 245)
n (%)Placebo
(N = 248)
n (%)COSENTYX
300 mg
(N = 327)
n (%)COSENTYX
150 mg
(N = 327)
n (%)Placebo
(N = 326)
n (%)PASI 75 response 200 (82) 174 (71) 11 (4) 249 (76) 219 (67) 16 (5) IGA of clear or almost clear 160 (65) 125 (51) 6 (2) 202 (62) 167 (51) 9 (3) The results of Trials 3 and 4 are presented in Table 3.
Table 3: Clinical Outcomes at Week 12 in Adults with Plaque Psoriasis in Trials 3 and 4 Trial 3 Trial 4 COSENTYX
300 mg
(N = 59)
n (%)COSENTYX
150 mg
(N = 59)
n (%)Placebo
(N = 59)
n (%)COSENTYX
300 mg
(N = 60)
n (%)COSENTYX
150 mg
(N = 61)
n (%)Placebo
(N = 61)
n (%)PASI 75 response 44 (75) 41 (69) 0 (0) 52 (87) 43 (70) 2 (3) IGA of clear or almost clear 40 (68) 31 (53) 0 (0) 44 (73) 32 (52) 0 (0) Examination of age, gender, and race subgroups did not identify differences in response to COSENTYX among these subgroups. Based on post-hoc sub-group analyses in patients with moderate to severe psoriasis, patients with lower body weight and lower disease severity may achieve an acceptable response with COSENTYX 150 mg.
PASI 90 response at Week 12 was achieved with COSENTYX 300 mg and 150 mg compared to placebo in 59% (145/245) and 39% (95/245) versus 1% (3/248) of subjects, respectively (Trial 1) and 54% (175/327) and 42% (137/327) versus 2% (5/326) of subjects, respectively (Trial 2). Similar results were seen in Trials 3 and 4.
With continued treatment over 52 weeks, subjects in Trial 1 who were PASI 75 responders at Week 12 maintained their responses in 81% (161/200) of the subjects treated with COSENTYX 300 mg and in 72% (126/174) of subjects treated with COSENTYX 150 mg. Trial 1 subjects who were clear or almost clear on the IGA at Week 12 also maintained their responses in 74% (119/160) of subjects treated with COSENTYX 300 mg and in 59% (74/125) of subjects treated with COSENTYX 150 mg. Similarly in Trial 2, PASI 75 responders maintained their responses in 84% (210/249) of subjects treated with COSENTYX 300 mg and in 82% (180/219) of subjects treated with COSENTYX 150 mg. Trial 2 subjects who were clear or almost clear on the IGA also maintained their responses in 80% (161/202) of subjects treated with COSENTYX 300 mg and in 68% (113/167) of subjects treated with COSENTYX 150 mg.
Among the subjects who chose to participate (39%) in assessments of patient reported outcomes, improvements in signs and symptoms related to itching, pain, and scaling, at Week 12 compared to placebo (Trials 1 and 2) were observed using the Psoriasis Symptom Diary©.
Psoriasis Lesions of Scalp
A randomized, placebo-controlled study enrolled 102 subjects with moderate to severe psoriasis lesions of scalp, defined as having a Psoriasis Scalp Severity Index (PSSI) score of greater than or equal to 12, an IGA scalp only score of 3 or greater, and at least 30% of the scalp affected. In this study, 62% of subjects had at least 50% of scalp surface area affected. The proportions of subjects achieving an IGA scalp only score of 0 or 1 (clear or almost clear) were 56.9% and 5.9% for the COSENTYX 300 mg and the placebo groups, respectively.
14.2 Psoriatic Arthritis
The safety and efficacy of COSENTYX were assessed in 1999 patients, in 3 randomized, double-blind, placebo-controlled studies (PsA1, PsA2 and PsA3) in adult patients, age 18 years and older with active psoriatic arthritis (greater than or equal to 3 swollen and greater than or equal to 3 tender joints) despite non-steroidal anti-inflammatory drug (NSAID), corticosteroid or disease modifying anti-rheumatic drug (DMARD) therapy. Patients in these studies had a diagnosis of PsA of at least 5 years across all studies. At baseline, over 61% and 42% of the patients had enthesitis and dactylitis, respectively. Overall, 31% of patients discontinued previous treatment with anti-TNFα agents due to either lack of efficacy or intolerance. In addition, approximately 53% of patients from both studies had concomitant methotrexate (MTX) use. Patients with different subtypes of PsA were enrolled including polyarticular arthritis with no evidence of rheumatoid nodules (80%), asymmetric peripheral arthritis (63%), distal interphalangeal involvement (58%), spondylitis with peripheral arthritis (20%) and arthritis mutilans (7%).
PsA1 Study (NCT 01752634) evaluated 397 patients, who were treated with COSENTYX 75 mg, 150 mg or 300 mg subcutaneous treatment at Weeks 0, 1, 2, 3 and 4, followed by the same dose every 4 weeks. Patients receiving placebo were re-randomized to receive COSENTYX (either 150 mg or 300 mg every 4 weeks) at Week 16 or Week 24 based on responder status. The primary endpoint was the percentage of patients achieving an ACR20 response at Week 24.
PsA2 Study (NCT 01392326) evaluated 606 patients, who were treated with secukinumab 10 mg/kg, intravenous treatment (or placebo) at Weeks 0, 2, and 4, followed by either 75 mg or 150 mg subcutaneous COSENTYX treatment (or placebo) every 4 weeks. Patients receiving placebo were re-randomized to receive COSENTYX (either 75 mg or 150 mg every 4 weeks) at Week 16 or Week 24 based on responder status.
PsA3 Study (NCT 02404350) evaluated 996 patients, who were treated with COSENTYX 150 mg or 300 mg subcutaneous treatment at Weeks 0, 1, 2, 3 and 4 followed by the same dose every 4 weeks, or once every 4 weeks of COSENTYX 150 mg. Patients treated with placebo received COSENTYX, either 150 mg or 300 mg, s.c., per baseline randomization, at Week 16 or Week 24 based upon responder status. The primary endpoint was ACR20 response at Week 16 with the key secondary endpoint the change from baseline in modified Total Sharp Score (mTSS) at Week 24.
Clinical Response
In PsA1, patients treated with 150 mg or 300 mg COSENTYX demonstrated a greater clinical response including ACR20, ACR50, and ACR70 compared to placebo at Week 24 (Table 4). Responses were similar in patients regardless of concomitant methotrexate treatment. Responses were seen regardless of prior anti-TNFα exposure.
In patients with coexistent plaque psoriasis receiving COSENTYX (n = 99), the skin lesions of psoriasis improved with treatment, relative to placebo, as measured by the Psoriasis Area Severity Index (PASI).
Table 4: Responsesa in PsA1 Study at Week 16 and Week 24 a Patients who met escape criteria (less than 20% improvement in tender or swollen joint counts) at Week 16 were considered non-responders COSENTYX COSENTYX Placebo Difference from placebo (95% CI) 150 mg
(N = 100)300 mg
(N = 100)
(N = 98)COSENTYX
150 mgCOSENTYX
300 mgACR20 response Week 16 (%) 60 57 18 42
(30, 54)38
(26, 51)Week 24 (%) 51 54 15 36
(24, 48)39
(27, 51)ACR50 response Week 16 (%) 37 35 6 31
(21, 42)28
(18, 39)Week 24 (%) 35 35 7 28
(18, 38)28
(17, 38)ACR70 response Week 16 (%) 17 15 2 15
(7, 23)13
(5, 20)Week 24 (%) 21 20 1 20
(12, 28)19
(11, 27)The percentage of patients achieving ACR20 response by visit is shown in Figure 1. Patients on placebo who received COSENTYX without a loading regimen achieved similar ACR20 responses over time (data not shown).
Figure 1: Percent of Patients Achieving ACR 20 Responsea in PsA1 Study Through Week 24
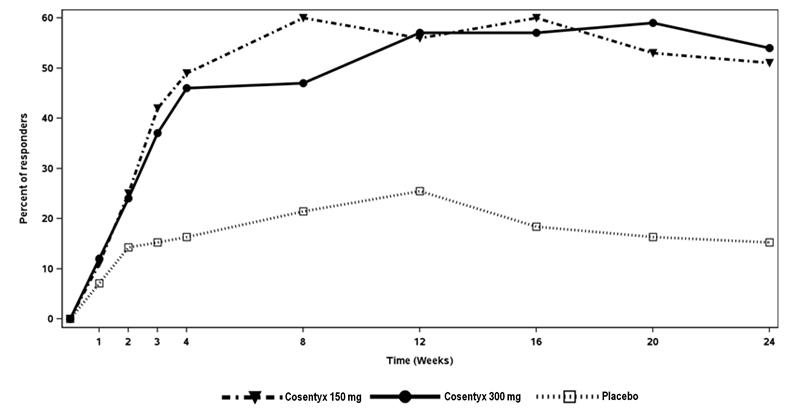
aPatients who met escape criteria (less than 20% improvement in tender or swollen joint counts) at Week 16 were considered non-responders
The improvements in the components of the ACR response criteria are shown in Table 5.
Table 5: Mean Change from Baseline in ACR Components at Week 16a (PsA1 Study) a Week 16 rather than Week 24 data are displayed to provide comparison between arms prior to placebo escape to COSENTYX.
b Mean Change based upon observed dataCOSENTYX
150 mg
(N = 100)COSENTYX
300 mg
(N = 100)Placebo
(N = 98)No. of Swollen Joints Baseline 12.0 11.2 12.1 Mean change at Week 16 -4.86 -5.83 -3.22 Number of Tender Joints Baseline 24.1 20.2 23.5 Mean change at Week 16 -10.70 -10.01 -1.77 Patient’s assessment of Pain Baseline 58.9 57.7 55.4 Mean change at Week 16 -22.91 -23.97 -7.98 Patient Global Assessment Baseline 62.0 60.7 57.6 Mean change at Week 16 -25.47 -25.40 -8.25 Physician Global Assessment Baseline 56.7 55.0 55.0 Mean change at Week 16 -29.24 -34.71 -14.95 Disability Index (HAQ) Baseline 1.2200 1.2828 1.1684 Mean change at Week 16 -0.45 -0.55 -0.23 CRP (mg/L) Baseline 14.15 10.88 7.87 Mean Change at Week 16b -8.41 -7.21 0.79 Improvements in enthesitis and dactylitis scores were observed in each COSENTYX group compared to placebo at Week 24.
Radiographic Response
In PsA3 Study, inhibition of progression of structural damage was assessed radiographically and expressed by the modified mTSS and its components, the Erosion Score (ES) and Joint Space Narrowing Score (JSN), at Week 24 compared to baseline. Radiographs of hands, wrists, and feet were obtained at baseline, Week 16 and/or Week 24 and scored independently by at least two readers who were blinded to treatment group and visit number. COSENTYX 150 mg without load, 150 mg with load and 300 mg with load treatment significantly inhibited progression of peripheral joint damage compared with placebo treatment as measured by change from baseline in mTSS at Week 24. The percentage of patients with no disease progression (defined as a change from baseline in mTSS of less than or equal to 0.0) from randomization to Week 24 was 75.7%, 70.9%, and 76.5% for COSENTYX 150 mg without load, 150 mg, 300 mg, respectively versus 68.2% for placebo.
Table 6: Rate of Change per 24 Weeks in Modified Total Sharp Score Results from a linear mixed effects model that excluded data after escape for placebo
subjects who received escape therapy at week 16. The model assumes approximately
linear progression over time and estimates a difference in rates (slopes) of progression
over 24 weeks to compare treatment arms.Treatment N Rate of Change per 24 weeks Difference from Placebo
(95% CI)COSENTYX 150 mg without load 210 -0.10 -0.61 (-0.95, -0.26) COSENTYX 150 mg with load 213 0.14 -0.37 (-0.71, -0.03) COSENTYX 300 mg with load 217 0.03 -0.48 (-0.82, -0.14) Placebo 296 0.51 -- Physical Function
Improvement in physical function as assessed by Health Assessment Questionnaire-Disability Index (HAQ-DI) demonstrated that the proportion of patients who achieved at least -0.3 improvement in HAQ-DI score from baseline was greater in the COSENTYX 150 mg and 300 mg groups compared to placebo at Week 16 and 24. At Week 16 in PsA1 study, estimated mean change from baseline was -0.23 in the placebo group compared with -0.45 in the COSENTYX 150 mg group and -0.55 in the COSENTYX 300 mg group.
14.3 Ankylosing Spondylitis
The safety and efficacy of COSENTYX were assessed in 816 patients in three randomized, double-blind, placebo-controlled studies (AS1, AS2 and AS3) in adult patients 18 years of age and older with active ankylosing spondylitis. Patients had active disease as defined by the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) greater or equal to 4 despite non-steroidal anti-inflammatory drug (NSAID), corticosteroid or disease modifying anti-rheumatic drug (DMARD) therapy. At baseline, approximately 13% and 25% used concomitant methotrexate or sulfasalazine, respectively. Overall, 29% of patients discontinued previous treatment with anti-TNFα agents due to either lack of efficacy or intolerance.
AS1 Study evaluated 219 patients, who were treated with COSENTYX 75 mg or 150 mg subcutaneous treatment at Weeks 0, 1, 2, 3 and 4, followed by the same dose every 4 weeks. At Week 16, patients receiving placebo were re-randomized to either COSENTYX 75 mg or 150 mg every 4 weeks. The primary endpoint was the percentage of patients achieving an ASAS20 response at Week 16.
AS2 Study evaluated 371 patients, who were treated with secukinumab 10 mg/kg intravenous treatment at Weeks 0, 2, and 4 (for both treatment arms) or placebo, followed by either 75 mg or 150 mg subcutaneous COSENTYX treatment every 4 weeks or placebo. Patients receiving placebo were re-randomized to receive COSENTYX (either 75 mg or 150 mg every 4 weeks) at Week 16 or Week 24 based on responder status.
AS3 Study evaluated 226 patients, who were treated with secukinumab 10 mg/kg intravenous treatment at Weeks 0, 2, and 4 (for both treatment arms) or placebo, followed by either 150 mg or 300 mg subcutaneous COSENTYX treatment every 4 weeks or placebo. Patients receiving placebo were re-randomized to receive COSENTYX (either 150 mg or 300 mg every 4 weeks) at Week 16. The primary endpoint was the percentage of patients achieving an ASAS20 response at Week 16. Patients were blinded to the treatment regimen up to Week 52, and the study continued to Week 156.
Clinical Response
In AS1, patients treated with 150 mg COSENTYX demonstrated greater improvements in ASAS20 and ASAS40 responses compared to placebo at Week 16 (Table 7). Responses were similar in patients regardless of concomitant therapies.
Table 7: ASAS20 and ASAS40 Responses in All AS Patients at Week 16 in Study AS1 COSENTYX
150 mg
(n = 72)Placebo
(n = 74)Difference from placebo
(95% CI)ASAS20 response, % 61 28 33
(18, 48)ASAS40 response, % 36 11 25
(12, 38)The improvements in the main components of the ASAS20 response criteria and other measures of disease activity are shown in Table 8.
Table 8: ASAS20 Components and Other Measures of Disease Activity at Week 16 (AS1 Study) - Percent of subjects with at least a 20% and 10 unit improvement measured on a Visual Analog Scale (VAS) with 0= none, 100= severe
- Bath Ankylosing Spondylitis Functional Index
- Inflammation is the mean of two patient-reported stiffness self-assessment in BASDAI
- Bath Ankylosing Spondylitis Disease Activity Index
- Bath Ankylosing Spondylitis Metrology Index
- High sensitivity C-reactive protein / mean change based upon observed data
COSENTYX
150 mg
(N = 72)Placebo
(N = 74)Baseline Week 16
change from baselineBaseline Week 16
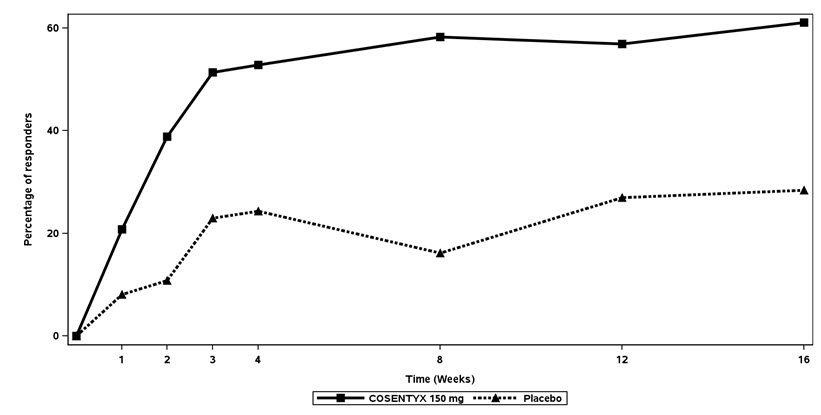
change from baselineASAS20 Response criteria -Patient Global Assessment of Disease Activity (0-100 mm)1 67.5 -27.7 70.5 -12.9 -Total spinal pain (0-100 mm) 66.2 -28.5 69.2 -10.9 -BASFI (0-10)2 6.2 -2.2 6.1 -0.7 -Inflammation (0-10)3 6.5 -2.5 6.5 -0.8 BASDAI Score4 6.6 -2.2 6.8 -0.9 BASMI5 3.6 -0.51 3.9 -0.22 hsCRP6 (mg/L) Mean Change at Week 16 27.0 -17.2 15.9 0.8 The percent of patients achieving ASAS20 responses by visit is shown in Figure 2. Patients on placebo who received COSENTYX without a loading regimen achieved similar ASAS20 responses over time (data not shown).
Figure 2: ASAS20 Responses in all AS1 Study Patients Over Time Up to Week 16
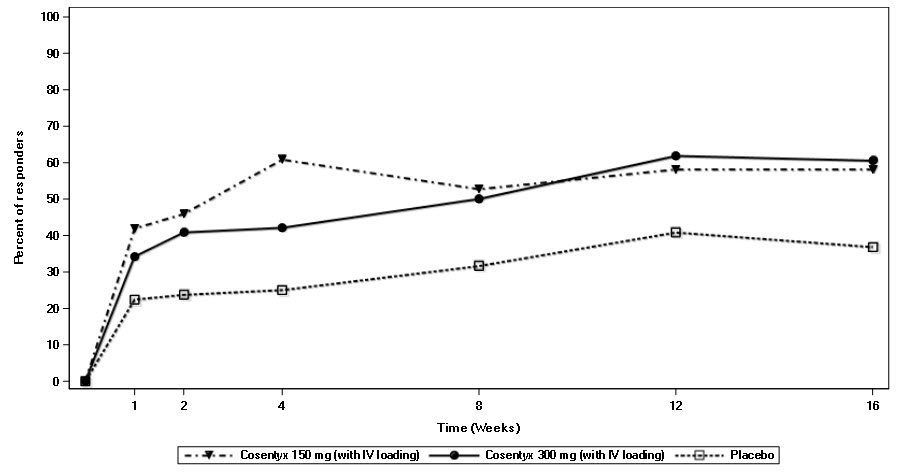
In AS3 Study, patients treated with COSENTYX (150 mg and 300 mg) demonstrated improved signs and symptoms, and had comparable efficacy responses, regardless of dose, that were superior to placebo at Week 16 for the primary and most secondary endpoints. At Week 16, the ASAS20 and ASAS40 responses were 58.1% and 40.5% for 150 mg and 60.5% and 42.1% for 300 mg, respectively. The percent of patients achieving ASAS20 responses by visit is shown in Figure 3.
Figure 3: ASAS20 Responses in all AS3 Study Patients Over Time Up to Week 16
COSENTYX treated patients showed improvement compared to placebo-treated patients in health-related quality of life as assessed by ASQoL at Week 16.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
COSENTYX Sensoready pen:
- NDC: 0078-0639-41: Carton of two 150 mg/mL (300 mg dose) Sensoready pens (injection)
- NDC: 0078-0639-68: Carton of one 150 mg/mL single-use Sensoready pen (injection)
COSENTYX prefilled syringe:
- NDC: 0078-0639-98: Carton of two 150 mg/mL (300 mg dose) single-use prefilled syringes (injection)
- NDC: 0078-0639-97: Carton of one 150 mg/mL single-use prefilled syringe (injection)
The removable cap of the COSENTYX Sensoready pen and prefilled syringe contains natural rubber latex. Each Sensoready pen and prefilled syringe is equipped with a needle safety guard.
COSENTYX vial (for healthcare professional use only):
- NDC: 0078-0657-61: Carton of one 150 mg lyophilized powder in a single-use vial (for injection)
16.2 Storage and Handling
COSENTYX Sensoready pens, prefilled syringes and vials must be refrigerated at 2ºC to 8ºC (36ºF to 46ºF). Keep the product in the original carton to protect from light until the time of use. Do not freeze. To avoid foaming do not shake. COSENTYX does not contain a preservative; discard any unused portion.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read FDA-approved patient labeling (Medication Guide and Instructions for Use).
Patient Counseling
Instruct patients to read the Medication Guide before starting COSENTYX therapy and to re-read the Medication Guide each time the prescription is renewed.
Advise patients of the potential benefits and risks of COSENTYX.
Infections
Inform patients that COSENTYX may lower the ability of their immune system to fight infections. Instruct patients of the importance of communicating any history of infections to the doctor and contacting their doctor if they develop any symptoms of infection [see Warnings and Precautions (5.1)].
Hypersensitivity
Advise patients to seek immediate medical attention if they experience any symptoms of serious hypersensitivity reactions [see Warnings and Precautions (5.4)].
Instruction on Injection Technique
Perform the first self-injection under the supervision of a qualified healthcare professional. If a patient or caregiver is to administer COSENTYX, instruct him/her in injection techniques and assess their ability to inject subcutaneously to ensure the proper administration of COSENTYX [see Medication Guide and Instructions for Use].
Instruct patients or caregivers in the technique of proper syringe and needle disposal, and advise them not to reuse these items. Instruct patients to inject the full amount of COSENTYX (1 or 2 subcutaneous injections of 150 mg) according to the directions provided in the Medication Guide and Instructions for Use. Dispose of needles, syringes and pens in a puncture-resistant container.
Manufactured by:
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey 07936
US License No. 1244© Novartis
T2020-09
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration Revised: January 2018 MEDICATION GUIDE
COSENTYX® (koe-sen-tix)
(secukinumab) InjectionWhat is the most important information I should know about COSENTYX?
COSENTYX is a medicine that affects your immune system. COSENTYX may increase your risk of having serious side effects such as:
Infections. COSENTYX may lower the ability of your immune system to fight infections and may increase your risk of infections.
Your healthcare provider should check you for tuberculosis (TB) before starting treatment with COSENTYX.
If your healthcare provider feels that you are at risk for TB, you may be treated with medicine for TB before you begin treatment with COSENTYX and during treatment with COSENTYX.
Your healthcare provider should watch you closely for signs and symptoms of TB during treatment with COSENTYX. Do not take COSENTYX if you have an active TB infection.
Before starting COSENTYX, tell your healthcare provider if you:
are being treated for an infection
have an infection that does not go away or that keeps coming back
have TB or have been in close contact with someone with TB
think you have an infection or have symptoms of an infection such as:o fever, sweats, or chills o warm, red, or painful skin or sores on your body o muscle aches o diarrhea or stomach pain o cough o burning when you urinate or urinate more often than normal o shortness of breath o blood in your phlegm o weight loss
After starting COSENTYX, call your healthcare provider right away if you have any of the signs of infection listed above. Do not use COSENTYX if you have any signs of infection unless you are instructed to by your healthcare provider.
See “What are the possible side effects of COSENTYX?” for more information about side effects.What is COSENTYX?
COSENTYX is a prescription medicine used to treat adults:- with moderate to severe plaque psoriasis that involves large areas or many areas of the body, and who may benefit from taking injections or pills (systemic therapy) or phototherapy (treatment using ultraviolet or UV light alone or with systemic therapy)
- with active psoriatic arthritis
- with active ankylosing spondylitis
It is not known if COSENTYX is safe and effective in children.Do not take COSENTYX:
Do not use COSENTYX if you have had a severe allergic reaction to secukinumab or any of the other ingredients in COSENTYX. See the end of this Medication Guide for a complete list of ingredients in COSENTYX.Before taking COSENTYX, tell your healthcare provider about all of your medical conditions, including if you: have any of the conditions or symptoms listed in the section “What is the most important information I should know about COSENTYX?”
have inflammatory bowel disease (Crohn’s disease or ulcerative colitis)
are allergic to latex. The needle cap on the COSENTYX Sensoready® pen and prefilled syringe contains latex.
have recently received or are scheduled to receive an immunization (vaccine). People who take COSENTYX should not receive live vaccines.
have any other medical conditions
are pregnant or plan to become pregnant. It is not known if COSENTYX can harm your unborn baby. You and your healthcare provider should decide if you will use COSENTYX.
are breastfeeding or plan to breastfeed. It is not known if COSENTYX passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of your medicines to show your healthcare provider and pharmacist when you get a new medicine.How should I use COSENTYX?
See the detailed “Instructions for Use” that comes with your COSENTYX for information on how to prepare and inject a dose of COSENTYX, and how to properly throw away (dispose of) used COSENTYX Sensoready pens and prefilled syringes.
Use COSENTYX exactly as prescribed by your healthcare provider.
If your healthcare provider decides that you or a caregiver may give your injections of COSENTYX at home, you should receive training on the right way to prepare and inject COSENTYX. Do not try to inject COSENTYX yourself, until you or your caregiver has been shown how to inject COSENTYX by your healthcare provider.
COSENTYX comes in a Sensoready pen or prefilled syringe that you or your caregiver may use at home to give injections. Your healthcare provider will decide which type of COSENTYX is best for you to use at home.
Your healthcare provider will prescribe the dose of COSENTYX that is right for you.
o If your prescribed dose of COSENTYX is 150 mg, you must give 1 injection of COSENTYX for each dose.
o If your prescribed dose of COSENTYX is 300 mg, you must give 2 injections for each dose.
COSENTYX is given as an injection under your skin (subcutaneous injection), in your upper legs (thighs) or stomach-area (abdomen) by you or a caregiver. A caregiver may also give you an injection of COSENTYX in your upper outer arm.
Do not give an injection in an area of the skin that is tender, bruised, red or hard, or in an area of skin that is affected by psoriasis.
Each injection should be given at a different site. Do not use the 2-inch area around your navel (belly button).
If you inject more COSENTYX than prescribed, call your healthcare provider or go to the nearest emergency room right away.What are the possible side effects of COSENTYX?
See “What is the most important information I should know about COSENTYX?”
Inflammatory bowel disease. New cases of inflammatory bowel disease or “flare-ups” can happen with COSENTYX, and can sometimes be serious. If you have inflammatory bowel disease (ulcerative colitis or Crohn’s disease), tell your healthcare provider if you have worsening disease symptoms during treatment with COSENTYX or develop new symptoms of stomach pain or diarrhea.
Serious allergic reactions. Get emergency medical help right away if you get any of the following symptoms of a serious allergic reaction:
o feel faint
o swelling of your face, eyelids, lips, mouth, tongue, or throat
o trouble breathing or throat tightness
o chest tightness
o skin rash
If you have a severe allergic reaction, do not give another injection of COSENTYX.
The most common side effects of COSENTYX include:
cold symptoms
diarrhea
upper respiratory infections
These are not all of the possible side effects of COSENTYX.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store COSENTYX?
Store COSENTYX in a refrigerator, between 36°F to 46°F (2°C to 8°C).
Keep COSENTYX in the original carton until ready for use to protect from light.
Do not freeze COSENTYX.
Do not shake COSENTYX.
Keep COSENTYX and all medicines out of the reach of children.General information about the safe and effective use of COSENTYX.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use COSENTYX for a condition for which it was not prescribed. Do not give COSENTYX to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about COSENTYX that is written for health professionals.What are the ingredients in COSENTYX?
Active ingredient: secukinumab
Inactive ingredients: Sensoready pen and prefilled syringe: L-histidine/histidine hydrochloride monohydrate, L-methionine, polysorbate 80, trehalose dihydrate, and sterile water for injection.
Vial: L-histidine/histidine hydrochloride monohydrate, polysorbate 80, and sucrose.
Manufactured by: Novartis Pharmaceuticals Corporation East Hanover, New Jersey 07936
T2018-06
For more information, call 1-888-669-6682 or go to www.COSENTYX.com -
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
COSENTYX® (koe-sen-tix)
(secukinumab)
For Injection
The following information is intended for medical or healthcare professionals only.
IMPORTANT:
- The single-use vial contains 150 mg of COSENTYX for reconstitution with Sterile Water for Injection (SWFI). Do not use the vial after the expiry date shown on the outer box or vial. If it has expired, return the entire pack to the pharmacy.
- The preparation of the solution for subcutaneous injection shall be done without interruption ensuring that aseptic technique is used. The preparation time from piercing the stopper until end of reconstitution on average takes 20 minutes and should not exceed 90 minutes.
- Throw away (dispose of) the used syringe right away after use. Do not re-use a syringe. See “How should I dispose of a used syringe?” at the end of this Instructions for Use.
How should I store COSENTYX?
- Store the vial of COSENTYX in the refrigerator between 2°C to 8°C (36°F to 46°F).
To prepare COSENTYX 150 mg for injection, please adhere to the following instructions:
Instructions for reconstitution of COSENTYX 150 mg for injection:
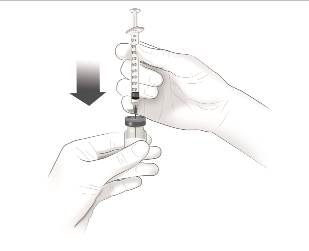
Step 1. Remove the vial of COSENTYX 150 mg for injection from the refrigerator and allow to stand for 15 to 30 minutes to reach room temperature. Ensure the Sterile Water for Injection (SWFI) is at room temperature.
Step 2. Reconstitute the lyophilized powder by slowly injecting 1 mL of Sterile Water for Injection (SWFI) into the vial. Direct the stream of SWFI onto the lyophilized powder (See Figure A).Figure A
Step 3. Tilt the vial to an angle of approximately 45 degrees and gently rotate between the fingertips for approximately 1 minute. Do not shake or invert the vial (See Figure B).
Step 4. Keep the vial standing at room temperature for a minimum of 10 minutes to allow for dissolution. Note that foaming of the solution may occur.
Step 5. Tilt the vial to an angle of approximately 45 degrees and gently rotate between the fingertips for approximately 1 minute. Do not shake or invert the vial (See Figure B).
Step 6. Allow the vial to stand undisturbed at room temperature for approximately 5 minutes. The resulting solution should be clear. Its color may vary from colorless to slightly yellow. Do not use if the lyophilized powder has not fully dissolved or if the liquid contains visible particles, is cloudy or is discolored.
Step 7. Prepare the required number of vials (1 vial for the 150 mg dose or 2 vials for the 300 mg dose).Figure B
After preparation, use the solution for subcutaneous injection immediately or store at 2°C to 8°C (36°F to 46°F) for up to 24 hours. Do not freeze. After storage at 2°C to 8°C (36°F to 46°F), allow the reconstituted solution to come to room temperature (15 to 30 minutes) before administration. Administer the solution within 1 hour after removal from the 2°C to 8°C (36°F to 46°F) storage.
Instructions for administration of COSENTYX solution:Step 1. Tilt the vial to an angle of approximately 45 degrees and position the needle tip at the very bottom of the solution in the vial when drawing the solution into the syringe. DO NOT invert the vial.
Step 2. Carefully withdraw slightly more than 1 mL of the solution for subcutaneous injection from the vial into a 1 mL graduated disposable syringe using a suitable needle (e.g., 21G x 2”) (See Figure C). This needle will only be used for withdrawing COSENTYX into the disposable syringe. Prepare the required number of syringes (1 syringe for the 150 mg dose or 2 syringes for the 300 mg dose).Figure C
Step 3. With the needle pointing upward, gently tap the syringe to move any air bubbles to the top (See Figure D). Figure D
Step 4. Replace the attached needle with a 27G x ½” needle (See Figure E).
Step 5. Expel the air bubbles and advance the plunger to the 1 mL mark.
Step 6. Clean the injection site with an alcohol wipe.Figure E

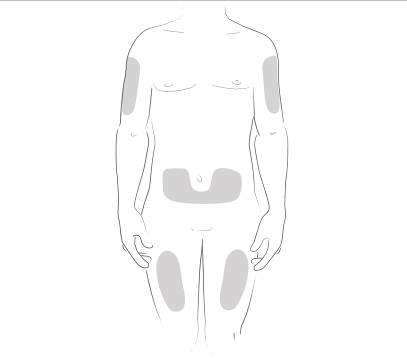
Step 7. Inject the COSENTYX solution subcutaneously into the front of thighs, lower abdomen [but not the area 2 inches around the navel (belly button)] or outer upper arms (See Figure F). Choose a different site each time an injection is administered. Do not inject into areas where the skin is tender, bruised, red, scaly or hard, or in an area of skin that is affected by psoriasis. Avoid areas with scars or stretch marks. Figure F
How should I dispose of a used syringe?
Any remaining solution in the vial must not be used and must be discarded in accordance with local requirements. Vials are for single use only.
Put the used syringes and needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) the syringes and needles in your household trash.
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey 07936
US License Number 1244
Revised: January 2018
© Novartis
T2018-07 -
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
COSENTYX® (koe-sen-tix)
(secukinumab)
Injection
Prefilled Syringe
Be sure that you read, understand, and follow this Instructions for Use before injecting COSENTYX. Your healthcare provider should show you how to prepare and inject COSENTYX properly using the prefilled syringe before you use it for the first time. Talk to your healthcare provider if you have any questions.
Important:
- Do not use the COSENTYX prefilled syringe if either the seal on the outside carton or the seal of the blister are broken. Keep the COSENTYX prefilled syringe in the sealed carton until you are ready to use it.
- Inject COSENTYX within 1 hour after taking it out of the refrigerator.
- Do not shake the COSENTYX prefilled syringe.
- The needle caps of the prefilled syringes contain latex. Do not handle the prefilled syringes if you are sensitive to latex.
- The prefilled syringe has a needle guard that will be activated to cover the needle after the injection is finished. The needle guard will help to prevent needle stick injuries to anyone who handles the prefilled syringe.
- Do not remove the needle cap until just before you give the injection.
- Avoid touching the syringe guard wings before use. Touching them may cause the syringe guard to be activated too early.
- Throw away (dispose of) the used COSENTYX prefilled syringe right away after use. Do not re-use a COSENTYX prefilled syringe. See “How should I dispose of used COSENTYX prefilled syringes?” at the end of this Instructions for Use.
How should I store COSENTYX?
- Store your carton of COSENTYX prefilled syringes in a refrigerator, between 36°F to 46°F (2°C to 8°C).
- Keep COSENTYX prefilled syringes in the original carton until ready to use to protect from light.
- Do not freeze COSENTYX prefilled syringes.
Keep COSENTYX and all medicines out of the reach of children.
COSENTYX prefilled syringe parts (see Figure A):
Figure A 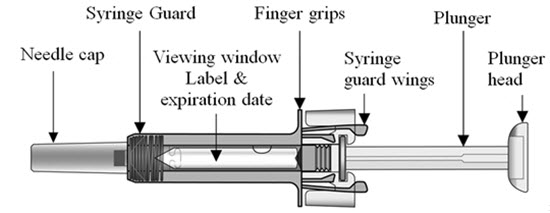
What you need for your injection:
Included in the carton: A new COSENTYX prefilled syringe. Each COSENTYX prefilled syringe contains 150 mg of COSENTYX.
- If your prescribed dose of COSENTYX is 150 mg, you must give 1 injection.
- If your prescribed dose of COSENTYX is 300 mg, you must give 2 injections.
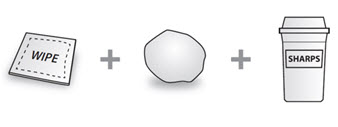
Not included in the carton (see Figure B):
1 Alcohol wipe
1 Cotton ball or gauze
Sharps disposal container
See “How should I dispose of used COSENTYX prefilled syringes?” at the end of this Instructions for Use.
Figure B
Prepare the COSENTYX prefilled syringe Step 1. Find a clean, well-lit, flat work surface. Step 2. Take the carton containing the COSENTYX prefilled syringe out of the refrigerator and leave it unopened on your work surface for about 15 to 30 minutes so that it reaches room temperature. Step 3. Wash your hands well with soap and water. Step 4. Remove the COSENTYX prefilled syringe from the outer carton and take it out of the blister. Step 5. Look through the viewing window on the COSENTYX prefilled syringe. The liquid inside should be clear. The color may be colorless to slightly yellow. You may see a small air bubble in the liquid. This is normal. Do not use the prefilled syringe if the liquid contains visible particles, or if the liquid is cloudy or discolored. Step 6. Do not use the COSENTYX prefilled syringe if it is broken. Return the prefilled syringe and the package it came in to the pharmacy. Step 7. Do not use the COSENTYX prefilled syringe if the expiration date has passed.
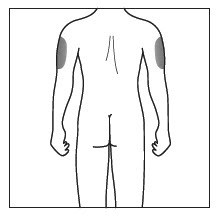
Choose and clean the injection site - Areas of your body that you may use as injection sites include:
- the front of your thighs (see Figure C)
- the lower stomach-area (abdomen), but not the area 2 inches around your navel (belly button) (see Figure C)
- your upper outer arms, if a caregiver is giving you the injection (see Figure D)
- Choose a different site for each injection of COSENTYX.
- Do not inject into areas where the skin is tender, bruised, red, scaly, or hard, or in an area of skin that is affected by psoriasis. Avoid areas with scars or stretch marks.
Figure C
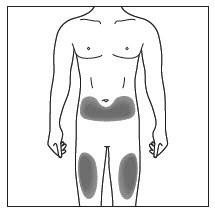
Step 8. Using a circular motion, clean the injection site with the alcohol wipe. Leave it to dry before injecting. Do not touch the cleaned area again before injecting. Figure D
Giving your injectionStep 9. Carefully remove the needle cap from the COSENTYX prefilled syringe (see Figure E). Throw away the needle cap. You may see a drop of liquid at the end of the needle. This is normal. Figure E
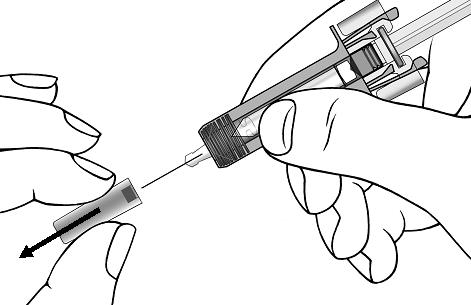
Step 10. With one hand gently pinch the skin at the injection site. With your other hand insert the needle into your skin as shown (see Figure F). Push the needle all the way in to make sure that you inject your full dose. Figure F
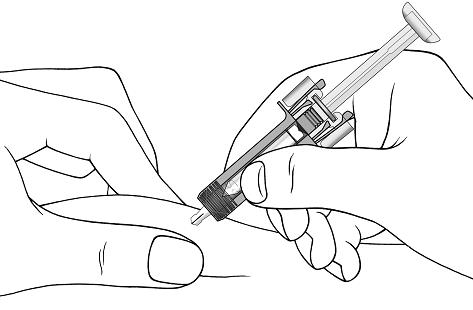
Step 11. Hold the COSENTYX prefilled syringe finger grips as shown (see Figure G). Slowly press down on the plunger as far as it will go, so that the plunger head is completely between the syringe guard wings.
Step 12. Continue to press fully on the plunger for an additional 5 seconds. Hold the syringe in place for the full 5 seconds.Figure G
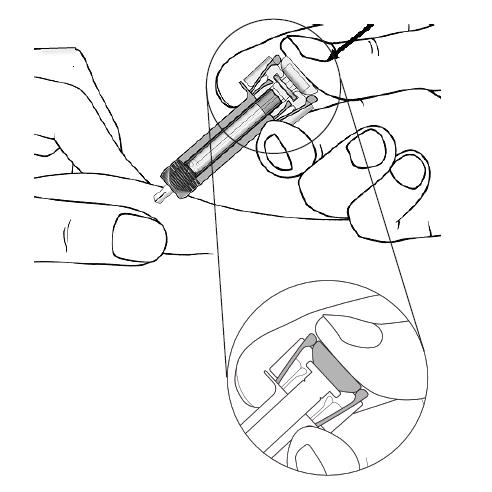
Step 13. Keep the plunger fully depressed while you carefully pull the needle straight out from the injection site (see Figure H). Figure H
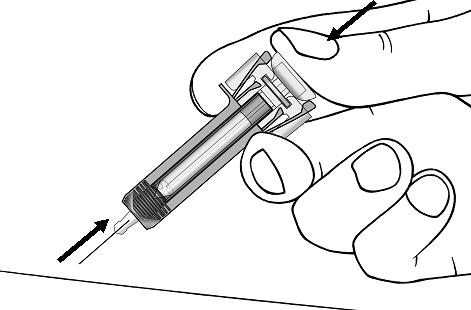
Step 14. Slowly release the plunger and allow the syringe guard to automatically cover the exposed needle (see Figure I).
Step 15. There may be a small amount of blood at the injection site. You can press a cotton ball or gauze over the injection site and hold it for 10 seconds. Do not rub the injection site. You may cover the injection site with a small adhesive bandage, if needed.Figure I
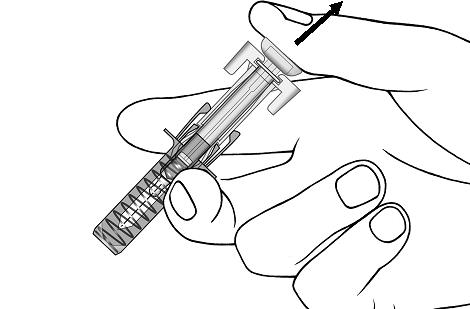
If your prescribed dose of COSENTYX is 300 mg, repeat steps 4 through 15 with a new COSENTYX prefilled syringe.
How should I dispose of used COSENTYX prefilled syringes?
Step 16. Put your used prefilled syringes in a FDA-cleared sharps disposal container right away after use (see Figure J). Do not throw away (dispose of) prefilled syringes in your household trash.
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles, syringes and prefilled syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.Figure J
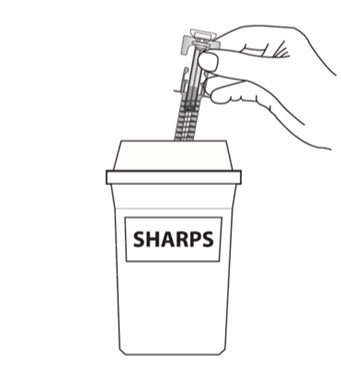
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey 07936
US License Number 1244
Revised: January 2018
© Novartis
T2018-08 -
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
COSENTYX® (koe-sen-tix)
(secukinumab)
Injection
Sensoready® Pen
Be sure that you read, understand, and follow this Instructions for Use before injecting COSENTYX. Your healthcare provider should show you how to prepare and inject COSENTYX properly using the Sensoready Pen before you use it for the first time. Talk to your healthcare provider if you have any questions.
Important:
- Do not use the COSENTYX Sensoready Pen if either the seal on the outer carton or the seal on the pen is broken. Keep the COSENTYX Sensoready Pen in the sealed outer carton until you are ready to use it.
- Inject COSENTYX within 1 hour after taking it out of the refrigerator.
- Do not shake the COSENTYX Sensoready Pen.
- The caps of the Sensoready Pens contain latex. Do not handle the Sensoready Pens if you are sensitive to latex.
- If you drop your COSENTYX Sensoready Pen, do not use it if the Sensoready Pen looks damaged, or if you dropped it with the cap removed.
- Throw away (dispose of) the used COSENTYX Sensoready Pen right away after use. Do not re-use a COSENTYX Sensoready Pen. See “How should I dispose of used COSENTYX Sensoready Pens?” at the end of this Instructions for Use.
How should I store COSENTYX?
- Store your carton of COSENTYX Sensoready Pen in a refrigerator, between 36°F to 46°F (2°C to 8°C).
- Keep COSENTYX Sensoready Pen in the original carton until ready to use to protect from light.
- Do not freeze COSENTYX Sensoready Pen.
Keep COSENTYX and all medicines out of the reach of children.
COSENTYX Sensoready Pen parts (see Figure A): Figure A 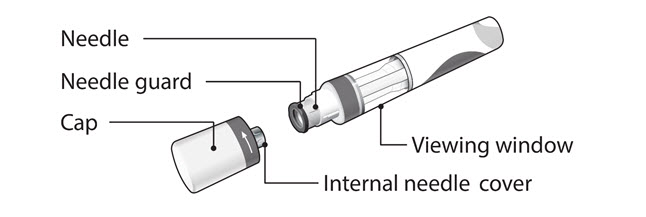
The COSENTYX Sensoready Pen is shown above with the cap removed. Do not remove the cap until you are ready to inject. What you need for your injection:
Included in the carton:
A new COSENTYX Sensoready Pen (see Figure B).
Each COSENTYX Sensoready Pen contains 150 mg of COSENTYX.- If your prescribed dose of COSENTYX is 150 mg, you must give 1 injection.
- If your prescribed dose of COSENTYX is 300 mg, you must give 2 injections.
Figure B
Not included in the carton (see Figure C):
- 1 Alcohol wipe
- 1 Cotton ball or gauze
- Sharps disposal container.
Figure C
Before your injection:
Take the COSENTYX Sensoready Pen out of the refrigerator 15 to 30 minutes before injecting to allow it to reach room temperature.
Step 1. Important safety checks before you inject (see Figure D):
- Look through the viewing window. The liquid should be clear. Its color may vary from colorless to slightly yellow.
Do not use if the liquid contains visible particles, is cloudy or is discolored. You may see a small air bubble, which is normal. - Look at the expiration date (EXP) on your Sensoready Pen. Do not use your COSENTYX Sensoready Pen if the expiration date has passed.
Figure D
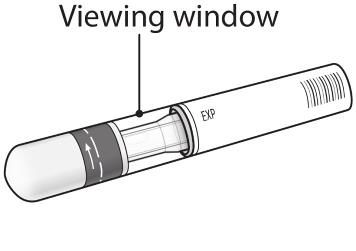
Step 2. Choose your injection site:
- The recommended site is the front of the thighs. You may also use the lower abdomen, but not the area 2 inches around your navel (belly button) (see Figure E).
- Choose a different site each time you give yourself an injection.
- Do not inject into areas where the skin is tender, bruised, red, scaly or hard, or in an area of skin that is affected by psoriasis. Avoid areas with scars or stretch marks.
Figure E
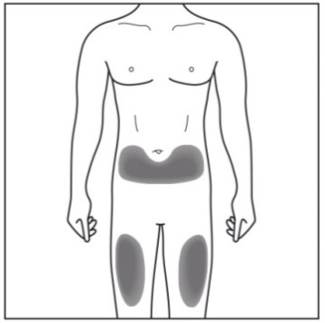
- If a caregiver or healthcare provider is giving you your injection, they may also inject into your outer upper arm (see Figure F).
Figure F
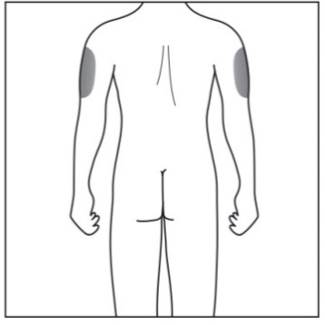
Step 3. Cleaning your injection site:
- Wash your hands well with soap and water.
- Using a circular motion, clean the injection site with the alcohol wipe. Leave it to dry before injecting (see Figure G).
- Do not touch the cleaned area again before injecting.
Figure G
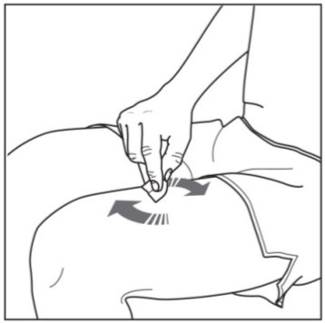
Your injection:
Step 4. Removing the cap:
- Only remove the cap when you are ready to use the COSENTYX Sensoready Pen.
- Twist off the cap in the direction of the arrow (see Figure H).
- Throw away the cap. Do not try to re-attach the cap.
- Use the COSENTYX Sensoready Pen within 5 minutes of removing the cap.
Figure H
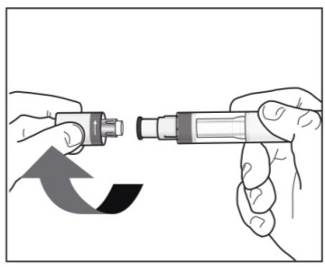
Step 5. Holding your COSENTYX Sensoready Pen:
- Hold the COSENTYX Sensoready Pen at 90 degrees to the cleaned injection site (see Figure I).
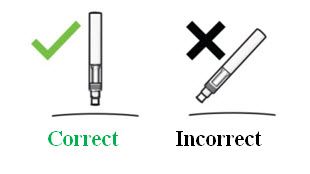
Figure I
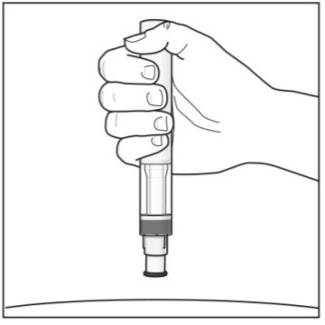
Important: During the injection you will hear 2 loud clicks: - The 1st click indicates that the injection has started.
- Several seconds later a 2nd click will indicate that the injection is almost finished.
Step 6. Starting your injection:
- Press the COSENTYX Sensoready Pen firmly against the skin to start the injection (see Figure J).
- The 1st click indicates the injection has started.
- Keep holding the COSENTYX Sensoready Pen firmly against your skin.
- The green indicator shows the progress of the injection.
Figure J
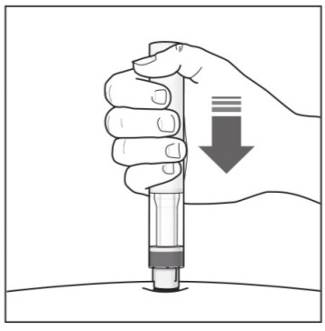
Step 7. Completing your injection:
- Listen for the 2nd click. This indicates the injection is almost complete.
- Check the green indicator fills the window and has stopped moving (see Figure K).
- The COSENTYX Sensoready Pen can now be removed.
Figure K
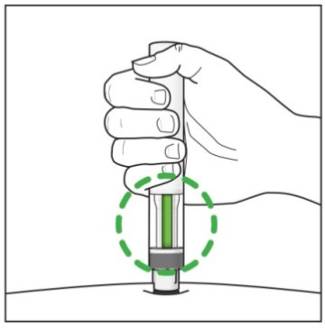
After your injection:
Step 8. Check the green indicator fills the window (see Figure L):
- This means the medicine has been delivered. Contact your healthcare provider if the green indicator is not visible.
- There may be a small amount of blood at the injection site. You can press a cotton ball or gauze over the injection site and hold it for 10 seconds. Do not rub the injection site. You may cover the injection site with a small adhesive bandage, if needed.
Figure L
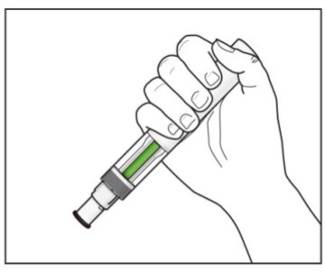
How should I dispose of used COSENTYX Sensoready Pens?
Step 9. Put your used Sensoready Pens in a FDA-cleared sharps disposal container right away after use (see Figure M). Do not throw away (dispose of) Sensoready Pens in your household trash.
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
Figure M
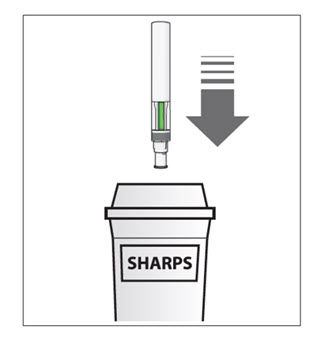
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey 07936
US License Number 1244
Revised: January 2018
© Novartis
T2018-09 -
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC: 0078-0639-97
Cosentyx®
(secukinumab)
Injection
Single-use Prefilled Syringe
150 mg/mL
1 Prefilled Syringe
ATTENTION: Dispense with enclosed Medication Guide.
For Subcutaneous Use Only
Sterile Solution - Contains No Preservative
Caution: Contains Natural Rubber Latex Which May Cause Allergic Reaction.
Rx only
NOVARTIS
-
INGREDIENTS AND APPEARANCE
COSENTYX
secukinumab injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0078-0639 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SECUKINUMAB (UNII: DLG4EML025) (SECUKINUMAB - UNII:DLG4EML025) SECUKINUMAB 150 mg in 1 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) HISTIDINE MONOHYDROCHLORIDE MONOHYDRATE (UNII: X573657P6P) METHIONINE (UNII: AE28F7PNPL) POLYSORBATE 80 (UNII: 6OZP39ZG8H) NITROGEN (UNII: N762921K75) TREHALOSE DIHYDRATE (UNII: 7YIN7J07X4) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-0639-41 2 mL in 1 CARTON; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 01/21/2015 2 NDC: 0078-0639-68 1 mL in 1 CARTON; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 01/21/2015 3 NDC: 0078-0639-98 2 mL in 1 CARTON; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 01/21/2015 4 NDC: 0078-0639-97 1 mL in 1 CARTON; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 01/21/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125504 01/21/2015 Labeler - Novartis Pharmaceuticals Corporation (002147023)
Trademark Results [COSENTYX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 COSENTYX 86734365 4979935 Live/Registered |
Novartis AG 2015-08-24 |
 COSENTYX 85804029 4716891 Live/Registered |
Novartis AG 2012-12-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.