CUPRIC CHLORIDE injection, solution
CUPRIC CHLORIDE by
Drug Labeling and Warnings
CUPRIC CHLORIDE by is a Prescription medication manufactured, distributed, or labeled by Archis Pharma LLC, RK Pharma INC.,, Immacule Lifesciences Private Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Cupric Chloride Injection, USP is a sterile, nonpyrogenic solution intended for use as an additive to intravenous solutions for total parenteral nutrition (TPN). Each mL of solution contains 1.07 mg cupric chloride, dihydrate and 9 mg sodium chloride.
The solution contains no bacteriostat, antimicrobial agent or added buffer. The pH is 2.0 (1.5 to 2.5); product may contain hydrochloric acid and/or sodium hydroxide for pH adjustment. The osmolarity is 0.327 mOsmol/mL (calc.).
Cupric chloride, USP is chemically designated cupric chloride, dihydrate (CuCl 22H 2O), a crystalline compound freely soluble in water.
Sodium Chloride, USP is chemically designated NaCl, a white crystalline compound freely soluble in water.
-
CLINICAL PHARMACOLOGY
Copper is an essential nutrient which serves as a cofactor for serum ceruloplasmin, an oxidase necessary for proper formation of the iron carrier protein, transferrin. Copper also helps maintain normal rates of red and white blood cell formation.
Providing copper during TPN helps prevent development of the following deficiency symptoms: Leukopenia, neutropenia, anemia, depressed ceruloplasmin levels, impaired transferrin formation, secondary iron deficiency and osteoporosis.
Normal serum copper values range from 80 to 163 mcg/dl (mean, approximately 110 mcg/dl). The serum copper level at which deficiency symptoms appear is not precisely defined. A serum value of 9 mcg copper/dl was reported for one TPN patient who received no copper. The daily turnover of copper through ceruloplasmin is approximately 0.5 mg. Excretion of copper is through the bile (80%), directly through the intestinal wall (16%) and in urine (4%).
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Direct intramuscular or intravenous injection of cupric chloride injection is contraindicated, as the acidic pH of the solution (2) may cause considerable tissue irritation.
Liver and/or biliary tract dysfunction may require omission or reduction of copper and manganese doses because these elements are primarily eliminated in the bile.
WARNING:This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired.
Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
-
PRECAUTIONS
General
Do not use unless the solution is clear and the seal is intact.Administration of zinc in the absence of copper may cause a decrease in serum copper levels.
Cupric chloride injection should only be used in conjunction with a pharmacy directed admixture program using aseptic technique in a laminar flow environment; it should be used promptly and in a single operation without any repeated penetrations. Solution contains no preservatives; discard unused portion immediately after admixture procedure is completed.
It is not recommended to administer copper to a patient with Wilson’s Disease, a genetic disease of copper metabolism.
Drug Interactions
Cupric ion may degrade ascorbic acid in total parenteral nutrition (TPN) solutions. In order to avoid this loss of ascorbate, multivitamin additives should be added to TPN solutions immediately prior to infusion. Alternatively, the multivitamin additive may be added to one container of TPN solution, followed by copper in a subsequent container.Laboratory Tests
Twice monthly serum assays for copper and/or ceruloplasmin are suggested for monitoring copper concentrations in long-term TPN patients. As ceruloplasmin is a cuproenzyme, ceruloplasmin assays may be depressed secondary to copper deficiency.Carcinogenesis, Mutagenesis, and Impairment of Fertility
Long-term animal studies to evaluate the carcinogenic potential of cupric chloride injection have not been performed, nor have studies been done to assess mutagenesis or impairment of fertility.Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when cupric chloride injection is administered to a nursing woman.Pediatric Use
( See DOSAGE AND ADMINISTRATION Section.) There are limited data in infants weighing less than 1500 grams.Pregnancy
Animal reproduction studies have not been conducted with cupric chloride. It is also not known whether cupric chloride can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Cupric chloride should be given to a pregnant woman only if clearly indicated.Geriatric Use
An evaluation of current literature revealed no clinical experience identifying differences in response between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy - ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
-
OVERDOSAGE
Copper toxicity can produce prostration, behavior change, diarrhea, progressive marasmus, hypotonia, photophobia and peripheral edema. Such symptoms have been reported with a serum copper level of 286 mcg/dl. Copper toxicity can also result in hemolysis and liver toxicity, including hepatic necrosis which may be fatal. D-penicillamine has been reported effective as an antidote.
-
DOSAGE AND ADMINISTRATION
Cupric Chloride Injection contains 0.4 mg copper/mL and is administered intravenously only after dilution. The additive should be diluted in a volume of fluid not less than 100 mL. For the adult receiving TPN, the suggested additive dosage is 0.5 to 1.5 mg copper/day (1.25 to 3.75 mL/day). For pediatric patients, the suggested additive dosage is 20 mcg copper/kg/day (0.05 mL/kg/day). Infants weighing less than 1500 gm may have increased requirements because of their low body reserves and increased requirements for growth.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. (See PRECAUTIONS.)
-
HOW SUPPLIED
Cupric Chloride Injection, USP 0.4 mg/mL is supplied as follows:
NDC 72819-234-06 10 mL Single-dose glass vial
NDC 72819-234-16 Carton of 25 x 10 mL Single-dose glass vials.
Storage: Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Distributed by:
Archis Pharma LLC
15 Corporate Place South, Suite 108
Piscataway, NJ 08854 U.S.ARev. 05/2024
PI-CUC-00
LEIA-297.00
10541 -
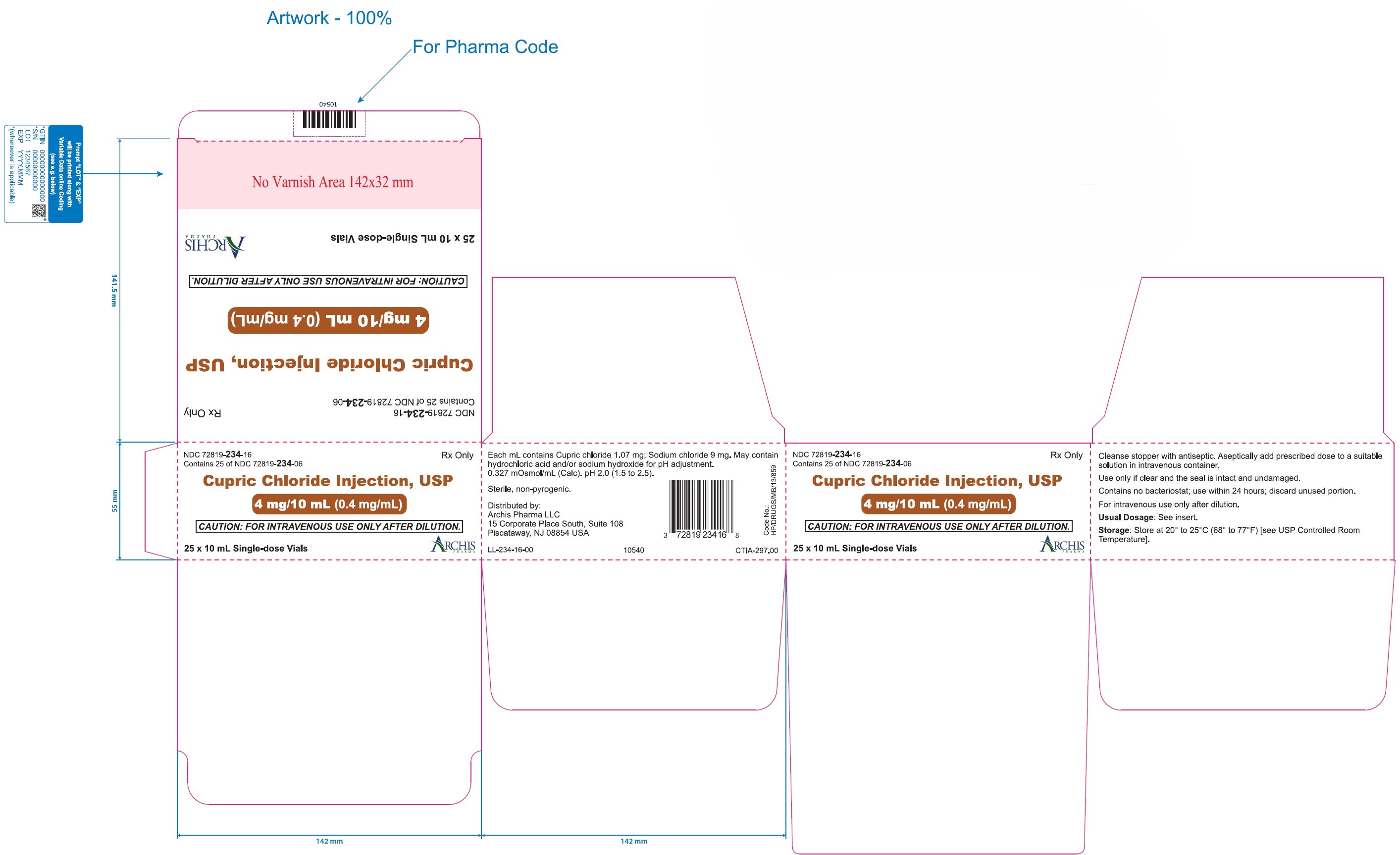
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
NDC 72819- 234-06
Cupric Chloride Injection, USP 4 mg/10 mL (0.4 mg/mL)
10 mL Single-dose Vials
Rx only

Principal Display Panel Text for Carton Label:
NDC 72819- 234-16
Cupric Chloride Injection, USP 4 mg/10 mL (0.4 mg/mL)
10 mL Single-dose vials in package of 25
Rx only
-
INGREDIENTS AND APPEARANCE
CUPRIC CHLORIDE
cupric chloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72819-234 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CUPRIC CHLORIDE (UNII: S2QG84156O) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 0.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 9 mg in 1 mL HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72819-234-16 25 in 1 PACKAGE 07/05/2024 1 NDC: 72819-234-06 10 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA217626 07/05/2024 Labeler - Archis Pharma LLC (026836212) Registrant - RK Pharma INC., (116901480) Establishment Name Address ID/FEI Business Operations Immacule Lifesciences Private Limited 650694503 manufacture(72819-234)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.