VORICONAZOLE- voriconazole tablet These highlights do not include all the information needed to use VORICONAZOLE safely and effectively. See full prescribing information for VORICONAZOLE. VORICONAZOLE Tablets for oral use. Initial U.S. Approval: 2002
Voriconazole by
Drug Labeling and Warnings
Voriconazole by is a Prescription medication manufactured, distributed, or labeled by APPCO PHARMA LLC, Kemwell Biopharma Private Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
VORICONAZOLE- voriconazole tablet
APPCO PHARMA LLC
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONVORICONAZOLE- voriconazole tablet
These highlights do not include all the information needed to use VORICONAZOLE safely and effectively. See full prescribing information for VORICONAZOLE. VORICONAZOLE Tablets for oral use. Initial U.S. Approval: 2002 RECENT MAJOR CHANGES
INDICATIONS AND USAGEVORICONAZOLE is an azole antifungal drug indicated for use in the treatment of:
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common adverse reactions (incidence ≥2%): visual disturbances, fever, nausea, rash, vomiting, chills, headache, liver function test abnormal, tachycardia, hallucinations (6)
To report SUSPECTED ADVERSE REACTIONS, contact Appco Pharma LLC at 1-855-672-7726 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 1/2016 |
|||||||||||||||||||||||||||||||||||
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
VORICONAZOLE is indicated for use in patients 12 years of age and older in the treatment of the following fungal infections:
1.1 Invasive Aspergillosis
In clinical trials, the majority of isolates recovered were Aspergillus fumigatus. There was a small number of cases of culture-proven disease due to species of Aspergillus other than A. fumigatus [see Clinical Studies (14.1), and Clinical Pharmacology (12.4)].
1.2 Candidemia in Non-neutropenic Patients and the Following Candida Infections: Disseminated Infections in Skin and Infections in Abdomen, Kidney, Bladder Wall, and Wounds
[see Clinical Studies (14.2), and Clinical Pharmacology (12.4)]
1.4 Serious Fungal Infections Caused by Scedosporium apiospermum (Asexual Form of Pseudallescheria boydii) and Fusarium spp. Including Fusarium solani, in Patients Intolerant of, or Refractory to, Other Therapy
[see Clinical Studies (14.4), and Clinical Pharmacology (12.4)]
Specimens for fungal culture and other relevant laboratory studies (including histopathology) should be obtained prior to therapy to isolate and identify causative organism(s). Therapy may be instituted before the results of the cultures and other laboratory studies are known. However, once these results become available, antifungal therapy should be adjusted accordingly.
2 DOSAGE AND ADMINISTRATION
2.1 Instructions for Use in All Patients
VORICONAZOLE Tablets should be taken at least one hour before or after a meal.
2.3 Recommended Dosing in Adults
Invasive aspergillosis and serious fungal infections due to Fusarium spp. and Scedosporium apiospermum
See Table 1. Therapy must be initiated with the specified loading dose regimen of intravenous VORICONAZOLE on Day 1 followed by the recommended maintenance dose regimen. Intravenous treatment should be continued for at least 7 days. Once the patient has clinically improved and can tolerate medication given by mouth, the oral tablet form or oral suspension form of VORICONAZOLE may be utilized. The recommended oral maintenance dose of 200 mg achieves a voriconazole exposure similar to 3 mg/kg IV; a 300 mg oral dose achieves an exposure similar to 4 mg/kg IV.
Switching between the intravenous and oral formulations is appropriate because of the high bioavailability of the oral formulation in adults [see Clinical Pharmacology (12)].
Candidemia in non-neutropenic patients and other deep tissue Candida infections
See Table 1. Patients should be treated for at least 14 days following resolution of symptoms or following last positive culture, whichever is longer.
Esophageal Candidiasis
See Table 1. Patients should be treated for a minimum of 14 days and for at least 7 days following resolution of symptoms.
|
|
|||
| Infection | Loading dose | Maintenance Dose *† | |
| IV | IV | Oral ‡ | |
| Invasive Aspergillosis § | 6 mg/kg q12h for the first 24 hours | 4 mg/kg q12h | 200 mg q12h |
| Candidemia in nonneutropenics and other deep tissue Candida infections | 6 mg/kg q12h for the first 24 hours | 3-4 mg/kg q12h ¶ | 200 mg q12h |
| Esophageal Candidiasis | # | # | 200 mg q12h |
| Scedosporiosis and Fusariosis | 6 mg/kg q12h for the first 24 hours | 4 mg/kg q12h | 200 mg q12h |
- Increase dose when VORICONAZOLE is co-administered with phenytoin or efavirenz (7); Decrease dose in patients with hepatic impairment (2.7)
- In healthy volunteer studies, the 200 mg oral q12h dose provided an exposure (AUCτ) similar to a 3 mg/kg IV q12h dose; the 300 mg oral q12h dose provided an exposure (AUCτ) similar to a 4 mg/kg IV q12h dose [see Clinical Pharmacology (12)].
- Adult patients who weigh less than 40 kg should receive half of the oral maintenance dose.
- In a clinical study of invasive aspergillosis, the median duration of IV Voriconazole therapy was 10 days (range 2-90 days). The median duration of oral Voriconazole therapy was 76 days (range 2-232 days) [see Clinical Studies (14.1)].
- In clinical trials, patients with candidemia received 3 mg/kg IV q12h as primary therapy, while patients with other deep tissue Candida infections received 4 mg/kg q12h as salvage therapy. Appropriate dose should be based on the severity and nature of the infection.
- Not evaluated in patients with esophageal candidiasis.
2.4 Dosage Adjustment
If patient response is inadequate, the oral maintenance dose may be increased from 200 mg every 12 hours (similar to 3 mg/kg IV q12h) to 300 mg every 12 hours (similar to 4 mg/kg IV q12h). For adult patients weighing less than 40 kg, the oral maintenance dose may be increased from 100 mg every 12 hours to 150 mg every 12 hours. If patient is unable to tolerate 300 mg orally every 12 hours, reduce the oral maintenance dose by 50 mg steps to a minimum of 200 mg every 12 hours (or to 100 mg every 12 hours for adult patients weighing less than 40 kg).
If patient is unable to tolerate 4 mg/kg IV q12h, reduce the intravenous maintenance dose to 3 mg/kg q12h.
The maintenance dose of voriconazole should be increased when co-administered with phenytoin or efavirenz [see Drug Interactions (7)].
The maintenance dose of voriconazole should be reduced in patients with mild to moderate hepatic impairment, Child-Pugh Class A and B [see Dosage and Administration (2.7)]. There are no PK data to allow for dosage adjustment recommendations in patients with severe hepatic impairment (Child-Pugh Class C).
Duration of therapy should be based on the severity of the patient’s underlying disease, recovery from immunosuppression, and clinical response.
2.7 Use in Patients With Hepatic Impairment
In the clinical program, patients were included who had baseline liver function tests (ALT, AST) up to 5 times the upper limit of normal. No dose adjustment is necessary in patients with this degree of abnormal liver function, but continued monitoring of liver function tests for further elevations is recommended [see Warnings and Precautions (5.9)].
It is recommended that the standard loading dose regimens be used but that the maintenance dose be halved in patients with mild to moderate hepatic cirrhosis (Child-Pugh Class A and B) [see Clinical Pharmacology (12.3)].
VORICONAZOLE has not been studied in patients with severe hepatic cirrhosis (Child-Pugh Class C) or in patients with chronic hepatitis B or chronic hepatitis C disease. VORICONAZOLE has been associated with elevations in liver function tests and clinical signs of liver damage, such as jaundice, and should only be used in patients with severe hepatic impairment if the benefit outweighs the potential risk. Patients with hepatic insufficiency must be carefully monitored for drug toxicity.
2.8 Use in Patients With Renal Impairment
The pharmacokinetics of orally administered VORICONAZOLE are not significantly affected by renal impairment. Therefore, no adjustment is necessary for oral dosing in patients with mild to severe renal impairment [see Clinical Pharmacology (12.3)] .
In patients with moderate or severe renal impairment (creatinine clearance <50 mL/min), accumulation of the intravenous vehicle, SBECD, occurs. Oral voriconazole should be administered to these patients, unless an assessment of the benefit/risk to the patient justifies the use of intravenous voriconazole. Serum creatinine levels should be closely monitored in these patients, and, if increases occur, consideration should be given to changing to oral voriconazole therapy [see Warnings and Precautions (5.10)].
Voriconazole is hemodialyzed with clearance of 121 mL/min. The intravenous vehicle, SBECD, is hemodialyzed with clearance of 55 mL/min. A 4-hour hemodialysis session does not remove a sufficient amount of voriconazole to warrant dose adjustment.
3 DOSAGE FORMS AND STRENGTHS
Tablets
VORICONAZOLE 50 mg tablets; white, round shaped, biconvex, film coated tablets with ‘APP 332’ debossed on one side and plain on other side.
VORICONAZOLE 200 mg tablets; white, oval shaped, biconvex, film coated tablets with ‘APP 333’ debossed on one side and plain on other side.
4 CONTRAINDICATIONS
- VORICONAZOLE is contraindicated in patients with known hypersensitivity to voriconazole or its excipients. There is no information regarding cross-sensitivity between VORICONAZOLE (voriconazole) and other azole antifungal agents. Caution should be used when prescribing VORICONAZOLE to patients with hypersensitivity to other azoles.
- Coadministration of terfenadine, astemizole, cisapride, pimozide or quinidine with VORICONAZOLE is contraindicated because increased plasma concentrations of these drugs can lead to QT prolongation and rare occurrences of torsade de pointes [see Drug Interactions (7), and Clinical Pharmacology (12.3)].
- Coadministration of VORICONAZOLE with sirolimus is contraindicated because VORICONAZOLE significantly increases sirolimus concentrations [see Drug Interactions (7), and Clinical Pharmacology (12.3)].
- Coadministration of VORICONAZOLE with rifampin, carbamazepine and long-acting barbiturates is contraindicated because these drugs are likely to decrease plasma voriconazole concentrations significantly [see Drug Interactions (7), and Clinical Pharmacology (12.3)].
- Coadministration of standard doses of voriconazole with efavirenz doses of 400 mg q24h or higher is contraindicated, because efavirenz significantly decreases plasma voriconazole concentrations in healthy subjects at these doses. Voriconazole also significantly increases efavirenz plasma concentrations [see Drug Interactions (7), and Clinical Pharmacology (12.3)].
- Coadministration of VORICONAZOLE with high-dose ritonavir (400 mg q12h) is contraindicated because ritonavir (400 mg q12h) significantly decreases plasma voriconazole concentrations. Coadministration of voriconazole and low-dose ritonavir (100 mg q12h) should be avoided, unless an assessment of the benefit/risk to the patient justifies the use of voriconazole [see Drug Interactions (7), and Clinical Pharmacology (12.3)].
- Coadministration of VORICONAZOLE with ergot alkaloids (ergotamine and dihydroergotamine) is contraindicated because VORICONAZOLE may increase the plasma concentration of ergot alkaloids, which may lead to ergotism [see Drug Interactions (7), Clinical Pharmacology (12.3)].
- Coadministration of VORICONAZOLE with St. John’s Wort is contraindicated because this herbal supplement may decrease voriconazole plasma concentration [see Drug Interactions (7), and Clinical Pharmacology (12.3)].
5 WARNINGS AND PRECAUTIONS
5.1 Drug Interactions
See Table 7 for a listing of drugs that may significantly alter voriconazole concentrations. Also, see Table 8 for a listing of drugs that may interact with voriconazole resulting in altered pharmacokinetics or pharmacodynamics of the other drug [ see Contraindications (4), and Drug Interactions (7) ].
5.2 Hepatic Toxicity
In clinical trials, there have been uncommon cases of serious hepatic reactions during treatment with VORICONAZOLE (including clinical hepatitis, cholestasis and fulminant hepatic failure, including fatalities). Instances of hepatic reactions were noted to occur primarily in patients with serious underlying medical conditions (predominantly hematological malignancy). Hepatic reactions, including hepatitis and jaundice, have occurred among patients with no other identifiable risk factors. Liver dysfunction has usually been reversible on discontinuation of therapy [see Warnings and Precautions (5.9), and Adverse Reaction (6.3)].
Measure serum transaminase levels and bilirubin at the initiation of VORICONAZOLE therapy and monitor at least weekly for the first month of treatment. Monitoring frequency can be reduced to monthly during continued use if no clinically significant changes are noted. If liver function tests become markedly elevated compared to baseline, VORICONAZOLE should be discontinued unless the medical judgment of the benefit-risk of the treatment for the patient justifies continued use [see Warnings and Precautions (5.9), Dosage and Administration (2.4,2.7), and Adverse Reactions (6.3)].
5.3 Visual Disturbances
The effect of VORICONAZOLE on visual function is not known if treatment continues beyond 28 days. There have been post-marketing reports of prolonged visual adverse events, including optic neuritis and papilledema. If treatment continues beyond 28 days, visual function including visual acuity, visual field and color perception should be monitored [ see Adverse Reactions(6.2)].
5.4 Embryo-Fetal Toxicity
Voriconazole can cause fetal harm when administered to a pregnant woman.
In animals, voriconazole administration was associated with teratogenicity, embryotoxicity, increased gestational length, dystocia and embryomortality. Please refer to section 8.1 (Use in Pregnancy) for additional details.
If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be informed of the potential hazard to the fetus.
5.5 Galactose Intolerance
VORICONAZOLE tablets contain lactose and should not be given to patients with rare hereditary problems of galactose intolerance, Lapp lactase deficiency or glucose-galactose malabsorption.
5.6 Arrhythmias and QT Prolongation
Some azoles, including voriconazole, have been associated with prolongation of the QT interval on the electrocardiogram. During clinical development and post-marketing surveillance, there have been rare cases of arrhythmias, (including ventricular arrhythmias such as torsade de pointes), cardiac arrests and sudden deaths in patients taking voriconazole. These cases usually involved seriously ill patients with multiple confounding risk factors, such as history of cardiotoxic chemotherapy, cardiomyopathy, hypokalemia and concomitant medications that may have been contributory.
Voriconazole should be administered with caution to patients with these potentially proarrhythmic conditions, such as:
- Congenital or acquired QT-prolongation
- Cardiomyopathy, in particular when heart failure is present
- Sinus bradycardia
- Existing symptomatic arrhythmias
- Concomitant medicinal product that is known to prolong QT interval [ Contraindications (4), Drug Interactions (7), and Clinical Pharmacology (12.3)]
Rigorous attempts to correct potassium, magnesium and calcium should be made before starting and during voriconazole therapy [see Clinical Pharmacology (12.3)] .
5.7 Infusion Related Reactions
During infusion of the intravenous formulation of voriconazole in healthy subjects, anaphylactoid-type reactions, including flushing, fever, sweating, tachycardia, chest tightness, dyspnea, faintness, nausea, pruritus and rash, have occurred uncommonly. Symptoms appeared immediately upon initiating the infusion. Consideration should be given to stopping the infusion should these reactions occur.
5.8 Laboratory Tests
Electrolyte disturbances such as hypokalemia, hypomagnesemia and hypocalcemia should be corrected prior to initiation of and during VORICONAZOLE therapy.
Patient management should include laboratory evaluation of renal (particularly serum creatinine) and hepatic function (particularly liver function tests and bilirubin).
5.9 Patients With Hepatic Impairment
It is recommended that the standard loading dose regimens be used but that the maintenance dose be halved in patients with mild to moderate hepatic cirrhosis (Child-Pugh Class A and B) receiving VORICONAZOLE [see Clinical Pharmacology (12.3), and Dosage and Administration (2.7)] .
VORICONAZOLE has not been studied in patients with severe cirrhosis (Child-Pugh Class C). VORICONAZOLE has been associated with elevations in liver function tests and clinical signs of liver damage, such as jaundice, and should only be used in patients with severe hepatic insufficiency if the benefit outweighs the potential risk. Patients with hepatic insufficiency must be carefully monitored for drug toxicity.
5.10 Patients With Renal Impairment
In patients with moderate to severe renal dysfunction (creatinine clearance <50 mL/min), accumulation of the intravenous vehicle, SBECD, occurs. Oral voriconazole should be administered to these patients, unless an assessment of the benefit/risk to the patient justifies the use of intravenous voriconazole. Serum creatinine levels should be closely monitored in these patients, and if increases occur, consideration should be given to changing to oral voriconazole therapy [see Clinical Pharmacology (12.3), and Dosage and Administration (2.8)] .
5.11 Monitoring of Renal Function
Acute renal failure has been observed in patients undergoing treatment with VORICONAZOLE. Patients being treated with voriconazole are likely to be treated concomitantly with nephrotoxic medications and have concurrent conditions that may result in decreased renal function.
Patients should be monitored for the development of abnormal renal function. This should include laboratory evaluation, particularly serum creatinine.
5.12 Monitoring of Pancreatic Function
Patients with risk factors for acute pancreatitis (e.g., recent chemotherapy, hematopoietic stem cell transplantation [HSCT]) should be monitored for the development of pancreatitis during VORICONAZOLE treatment.
5.13 Dermatological Reactions
Serious exfoliative cutaneous reactions, such as Stevens-Johnson syndrome, have been reported during treatment with VORICONAZOLE. If a patient develops an exfoliative cutaneous reaction, VORICONAZOLE should be discontinued.
VORICONAZOLE has been associated with photosensitivity skin reaction. Patients, including children, should avoid exposure to direct sunlight during VORICONAZOLE treatment and should use measures such as protective clothing and sunscreen with high sun protection factor (SPF). If phototoxic reactions occur, the patient should be referred to a dermatologist and VORICONAZOLE discontinuation should be considered. If VORICONAZOLE is continued despite the occurrence of phototoxicity- related lesions, dermatologic evaluation should be performed on a systematic and regular basis to allow early detection and management of premalignant lesions. Squamous cell carcinoma of the skin and melanoma have been reported during long-term VORICONAZOLE therapy in patients with photosensitivity skin reactions. If a patient develops a skin lesion consistent with premalignant skin lesions, squamous cell carcinoma or melanoma, VORICONAZOLE should be discontinued.
The frequency of phototoxicity reactions is higher in the pediatric population. Because squamous cell carcinoma has been reported in patients who experience photosensitivity reactions, stringent measures for photoprotection are warranted in children. In children experiencing photoaging injuries such as lentigines or ephelides, sun avoidance and dermatologic follow-up are recommended even after treatment discontinuation.
5.14 Skeletal Adverse Events
Fluorosis and periostitis have been reported during long-term voriconazole therapy. If a patient develops skeletal pain and radiologic findings compatible with fluorosis or periostitis, voriconazole should be discontinued [see Adverse Reactions (6.4)].
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.1 Overview
The most frequently reported adverse events (all causalities) in the therapeutic trials were visual disturbances (18.7%), fever (5.7%), nausea (5.4%), rash (5.3%), vomiting (4.4%), chills (3.7%), headache (3.0%), liver function test increased (2.7%), tachycardia (2.4%), hallucinations (2.4%). The treatment-related adverse events which most often led to discontinuation of voriconazole therapy were elevated liver function tests, rash, and visual disturbances [see Warning and Precautions (5.2, 5.3) , and Adverse Reactions (6.2, 6.3)].
6.2 Clinical Trial Experience in Adults
The data described in Table 3 reflect exposure to voriconazole in 1655 patients in the therapeutic studies. This represents a heterogeneous population, including immunocompromised compromised patients, e.g., patients with hematological malignancy or HIV and non-neutropenic patients. This subgroup does not include healthy subjects and patients treated in the compassionate use and non-therapeutic studies. This patient population was 62% male, had a mean age of 46 years (range 11-90, including 51 patients aged 12-18 years), and was 78% White and 10% Black. Five hundred sixty one patients had a duration of voriconazole therapy of greater than 12 weeks, with 136 patients receiving voriconazole for over six months. Table 3 includes all adverse events which were reported at an incidence of ≥2% during voriconazole therapy in the all therapeutic studies population, studies 307/602 and 608 combined, or study 305, as well as events of concern which occurred at an incidence of <2%.
In study 307/602, 381 patients (196 on voriconazole, 185 on amphotericin B) were treated to compare voriconazole to amphotericin B followed by other licensed antifungal therapy in the primary treatment of patients with acute invasive aspergillosis. The rate of discontinuation from voriconazole study medication due to adverse events was 21.4% (42/196 patients). In study 608, 403 patients with candidemia were treated to compare voriconazole (272 patients) to the regimen of amphotericin B followed by fluconazole (131 patients). The rate of discontinuation from voriconazole study medication due to adverse events was 19.5% out of 272 patients. Study 305 evaluated the effects of oral voriconazole (200 patients) and oral fluconazole (191 patients) in the treatment of esophageal candidiasis. The rate of discontinuation from voriconazole study medication in Study 305 due to adverse events was 7% (14/200 patients). Laboratory test abnormalities for these studies are discussed under Clinical Laboratory Values below.
|
|
||||||
| All
Therapeutic Studies | Studies 307/602 and 608
(IV/ oral therapy) | Study 305
(oral therapy) |
||||
| Voriconazole
N=1655 | Voriconazole
N=468 | Ampho B
†
N=185 | Ampho B→
Fluconazole N=131 | Voriconazole
N=200 | Fluconazole
N=191 |
|
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Special Senses ‡ | ||||||
| Abnormal vision | 310 (18.7) | 63 (13.5) | 1 (0.5) | 0 | 31 (15.5) | 8 (4.2) |
| Photophobia | 37 (2.2) | 8 (1.7) | 0 | 0 | 5 (2.5) | 2 (1.0) |
| Chromatopsia | 20 (1.2) | 2 (0.4) | 0 | 0 | 2 (1.0) | 0 |
| Body as a Whole | ||||||
| Fever | 94 (5.7) | 8 (1.7) | 25 (13.5) | 5 (3.8) | 0 | 0 |
| Chills | 61 (3.7) | 1 (0.2) | 36 (19.5) | 8 (6.1) | 1 (0.5) | 0 |
| Headache | 49 (3.0) | 9 (1.9) | 8 (4.3) | 1 (0.8) | 0 | 1 (0.5) |
| Cardiovascular System | ||||||
| Tachycardia | 39 (2.4) | 6 (1.3) | 5 (2.7) | 0 | 0 | 0 |
| Digestive System | ||||||
| Nausea | 89 (5.4) | 18 (3.8) | 29 (15.7) | 2 (1.5) | 2 (1.0) | 3 (1.6) |
| Vomiting | 72 (4.4) | 15 (3.2) | 18 (9.7) | 1 (0.8) | 2 (1.0) | 1 (0.5) |
| Liver function tests abnormal | 45 (2.7) | 15 (3.2) | 4 (2.2) | 1 (0.8) | 6 (3.0) | 2 (1.0) |
| Cholestatic jaundice | 17 (1.0) | 8 (1.7) | 0 | 1 (0.8) | 3 (1.5) | 0 |
| Metabolic and Nutritional Systems | ||||||
| Alkaline phosphatase increased | 59 (3.6) | 19 (4.1) | 4 (2.2) | 3 (2.3) | 10 (5.0) | 3 (1.6) |
| Hepatic enzymes increased | 30 (1.8) | 11 (2.4) | 5 (2.7) | 1 (0.8) | 3 (1.5) | 0 |
| SGOT increased | 31 (1.9) | 9 (1.9) | 0 | 1 (0.8) | 8 (4.0) | 2 (1.0) |
| SGPT increased | 29 (1.8) | 9 (1.9) | 1 (0.5) | 2 (1.5) | 6 (3.0) | 2 (1.0) |
| Hypokalemia | 26 (1.6) | 3 (0.6) | 36 (19.5) | 16 (12.2) | 0 | 0 |
| Bilirubinemia | 15 (0.9) | 5 (1.1) | 3 (1.6) | 2 (1.5) | 1 (0.5) | 0 |
| Creatinine increased | 4 (0.2) | 0 | 59 (31.9) | 10 (7.6) | 1 (0.5) | 0 |
| Nervous System | ||||||
| Hallucinations | 39 (2.4) | 13 (2.8) | 1 (0.5) | 0 | 0 | 0 |
| Skin and Appendages | ||||||
| Rash | 88 (5.3) | 20 (4.3) | 7 (3.8) | 1 (0.8) | 3 (1.5) | 1 (0.5) |
| Urogenital | ||||||
| Kidney function abnormal | 10 (0.6) | 6 (1.3) | 40 (21.6) | 9 (6.9) | 1 (0.5) | 1 (0.5) |
| Acute kidney failure | 7 (0.4) | 2 (0.4) | 11 (5.9) | 7 (5.3) | 0 | 0 |
Visual Disturbances
Voriconazole treatment-related visual disturbances are common. In therapeutic trials, approximately 21% of patients experienced abnormal vision, color vision change and/or photophobia. Visual disturbances may be associated with higher plasma concentrations and/or doses.
There have been post-marketing reports of prolonged visual adverse events, including optic neuritis and papilledema [see Warnings and Precautions (5.3)].
The mechanism of action of the visual disturbance is unknown, although the site of action is most likely to be within the retina. In a study in healthy subjects investigating the effect of 28-day treatment with voriconazole on retinal function, voriconazole caused a decrease in the electroretinogram (ERG) waveform amplitude, a decrease in the visual field, and an alteration in color perception. The ERG measures electrical currents in the retina. The effects were noted early in administration of voriconazole and continued through the course of study drug dosing. Fourteen days after end of dosing, ERG, visual fields and color perception returned to normal [see Warnings and Precautions (5.7)].
Dermatological Reactions
Dermatological reactions were common in the patients treated with voriconazole. The mechanism underlying these dermatologic adverse events remains unknown.
Serious cutaneous reactions, including Stevens-Johnson syndrome, toxic epidermal necrolysis and erythema multiforme have been reported during treatment with VORICONAZOLE. If a patient develops an exfoliative cutaneous reaction, VORICONAZOLE should be discontinued.
In addition, VORICONAZOLE has been associated with photosensitivity skin reactions. Patients should avoid strong, direct sunlight during VORICONAZOLE therapy. In patients with photosensitivity skin reactions, squamous cell carcinoma of the skin and melanoma have been reported during long-term therapy. If a patient develops a skin lesion consistent with squamous cell carcinoma or melanoma, VORICONAZOLE should be discontinued [see Warnings and Precautions (5.13)].
Less Common Adverse Events
The following adverse events occurred in <2% of all voriconazole-treated patients in all therapeutic studies (N=1655). This listing includes events where a causal relationship to voriconazole cannot be ruled out or those which may help the physician in managing the risks to the patients. The list does not include events included in Table 5 above and does not include every event reported in the voriconazole clinical program.
Body as a Whole: abdominal pain, abdomen enlarged, allergic reaction, anaphylactoid reaction [see Warnings and Precautions (5.6)], ascites, asthenia, back pain, chest pain, cellulitis, edema, face edema, flank pain, flu syndrome, graft versus host reaction, granuloma, infection, bacterial infection, fungal infection, injection site pain, injection site infection/inflammation, mucous membrane disorder, multi-organ failure, pain, pelvic pain, peritonitis, sepsis, substernal chest pain.
Cardiovascular: atrial arrhythmia, atrial fibrillation, AV block complete, bigeminy, bradycardia, bundle branch block, cardiomegaly, cardiomyopathy, cerebral hemorrhage, cerebral ischemia, cerebrovascular accident, congestive heart failure, deep thrombophlebitis, endocarditis, extrasystoles, heart arrest, hypertension, hypotension, myocardial infarction, nodal arrhythmia, palpitation, phlebitis, postural hypotension, pulmonary embolus, QT interval prolonged, supraventricular extrasystoles, supraventricular tachycardia, syncope, thrombophlebitis, vasodilatation, ventricular arrhythmia, ventricular fibrillation, ventricular tachycardia (including torsade de pointes) [see Warnings and Precautions (5.6)].
Digestive: anorexia, cheilitis, cholecystitis, cholelithiasis, constipation, diarrhea, duodenal ulcer perforation, duodenitis, dyspepsia, dysphagia, dry mouth, esophageal ulcer, esophagitis, flatulence, gastroenteritis, gastrointestinal hemorrhage, GGT/LDH elevated, gingivitis, glossitis, gum hemorrhage, gum hyperplasia, hematemesis, hepatic coma, hepatic failure, hepatitis, intestinal perforation, intestinal ulcer, jaundice, enlarged liver, melena, mouth ulceration, pancreatitis, parotid gland enlargement, periodontitis, proctitis, pseudomembranous colitis, rectal disorder, rectal hemorrhage, stomach ulcer, stomatitis, tongue edema.
Endocrine: adrenal cortex insufficiency, diabetes insipidus, hyperthyroidism, hypothyroidism.
Hemic and Lymphatic: agranulocytosis, anemia (macrocytic, megaloblastic, microcytic, normocytic), aplastic anemia, hemolytic anemia, bleeding time increased, cyanosis, DIC, ecchymosis, eosinophilia, hypervolemia, leukopenia, lymphadenopathy, lymphangitis, marrow depression, pancytopenia, petechia, purpura, enlarged spleen, thrombocytopenia, thrombotic thrombocytopenic purpura.
Metabolic and Nutritional: albuminuria, BUN increased, creatine phosphokinase increased, edema, glucose tolerance decreased, hypercalcemia, hypercholesteremia, hyperglycemia, hyperkalemia, hypermagnesemia, hypernatremia, hyperuricemia, hypocalcemia, hypoglycemia, hypomagnesemia, hyponatremia, hypophosphatemia, peripheral edema, uremia.
Musculoskeletal: arthralgia, arthritis, bone necrosis, bone pain, leg cramps, myalgia, myasthenia, myopathy, osteomalacia, osteoporosis.
Nervous System: abnormal dreams, acute brain syndrome, agitation, akathisia, amnesia, anxiety, ataxia, brain edema, coma, confusion, convulsion, delirium, dementia, depersonalization, depression, diplopia, dizziness, encephalitis, encephalopathy, euphoria, Extrapyramidal Syndrome, grand mal convulsion, Guillain-Barré syndrome, hypertonia, hypesthesia, insomnia, intracranial hypertension, libido decreased, neuralgia, neuropathy, nystagmus, oculogyric crisis, paresthesia, psychosis, somnolence, suicidal ideation, tremor, vertigo.
Respiratory System: cough increased, dyspnea, epistaxis, hemoptysis, hypoxia, lung edema, pharyngitis, pleural effusion, pneumonia, respiratory disorder, respiratory distress syndrome, respiratory tract infection, rhinitis, sinusitis, voice alteration.
Skin and Appendages: alopecia, angioedema, contact dermatitis, discoid lupus erythematosis, eczema, erythema multiforme, exfoliative dermatitis, fixed drug eruption, furunculosis, herpes simplex, maculopapular rash, melanoma, melanosis, photosensitivity skin reaction, pruritus, pseudoporphyria, psoriasis, skin discoloration, skin disorder, skin dry, Stevens-Johnson syndrome, squamous cell carcinoma, sweating, toxic epidermal necrolysis, urticaria.
Special Senses: abnormality of accommodation, blepharitis, color blindness, conjunctivitis, corneal opacity, deafness, ear pain, eye pain, eye hemorrhage, dry eyes, hypoacusis, keratitis, keratoconjunctivitis, mydriasis, night blindness, optic atrophy, optic neuritis, otitis externa, papilledema, retinal hemorrhage, retinitis, scleritis, taste loss, taste perversion, tinnitus, uveitis, visual field defect.
Urogenital: anuria, blighted ovum, creatinine clearance decreased, dysmenorrhea, dysuria, epididymitis, glycosuria, hemorrhagic cystitis, hematuria, hydronephrosis, impotence, kidney pain, kidney tubular necrosis, metrorrhagia, nephritis, nephrosis, oliguria, scrotal edema, urinary incontinence, urinary retention, urinary tract infection, uterine hemorrhage, vaginal hemorrhage.
6.3 Clinical Laboratory Values
The overall incidence of clinically significant transaminase abnormalities in all therapeutic studies was 12.4% (206/1655) of patients treated with voriconazole. Increased incidence of liver function test abnormalities may be associated with higher plasma concentrations and/or doses. The majority of abnormal liver function tests either resolved during treatment without dose adjustment or following dose adjustment, including discontinuation of therapy.
Voriconazole has been infrequently associated with cases of serious hepatic toxicity including cases of jaundice and rare cases of hepatitis and hepatic failure leading to death. Most of these patients had other serious underlying conditions.
Liver function tests should be evaluated at the start of and during the course of VORICONAZOLE therapy. Patients who develop abnormal liver function tests during VORICONAZOLE therapy should be monitored for the development of more severe hepatic injury. Patient management should include laboratory evaluation of hepatic function (particularly liver function tests and bilirubin). Discontinuation of VORICONAZOLE must be considered if clinical signs and symptoms consistent with liver disease develop that may be attributable to VORICONAZOLE [see Warnings and Precautions (5.2)].
Acute renal failure has been observed in severely ill patients undergoing treatment with VORICONAZOLE. Patients being treated with voriconazole are likely to be treated concomitantly with nephrotoxic medications and have concurrent conditions that may result in decreased renal function. It is recommended that patients are monitored for the development of abnormal renal function. This should include laboratory evaluation, particularly serum creatinine.Tables 4 to 6 show the number of patients with hypokalemia and clinically significant changes in renal and liver function tests in three randomized, comparative multicenter studies. In study 305, patients with esophageal candidiasis were randomized to either oral voriconazole or oral fluconazole. In study 307/602, patients with definite or probable invasive aspergillosis were randomized to either voriconazole or amphotericin B therapy. In study 608, patients with candidemia were randomized to either voriconazole or the regimen of amphotericin B followed by fluconazole.
| n = number of patients with a clinically significant abnormality while on study therapy
N = total number of patients with at least one observation of the given lab test while on study therapy ULN = upper limit of normal |
|||
|
|
|||
| Criteria * | Voriconazole | Fluconazole | |
| n/N (%) | n/N (%) | ||
| T. Bilirubin | >1.5x ULN | 8/185 (4.3) | 7/186 (3.8) |
| AST | >3.0x ULN | 38/187 (20.3) | 15/186 (8.1) |
| ALT | >3.0x ULN | 20/187 (10.7) | 12/186 (6.5) |
| Alk phos | >3.0x ULN | 19/187 (10.2) | 14/186 (7.5) |
| n = number of patients with a clinically significant abnormality while on study therapy
N = total number of patients with at least one observation of the given lab test while on study therapy ULN = upper limit of normal LLN = lower limit of normal |
|||
|
|
|||
| Criteria * | Voriconazole | Amphotericin B † | |
| n/N (%) | n/N (%) | ||
| T. Bilirubin | >1.5x ULN | 35/180 (19.4) | 46/173 (26.6) |
| AST | >3.0x ULN | 21/180 (11.7) | 18/174 (10.3) |
| ALT | >3.0x ULN | 34/180 (18.9) | 40/173 (23.1) |
| Alk phos | >3.0x ULN | 29/181 (16.0) | 38/173 (22.0) |
| Creatinine | >1.3x ULN | 39/182 (21.4) | 102/177 (57.6) |
| Potassium | <0.9x LLN | 30/181 (16.6) | 70/178 (39.3) |
| n = number of patients with a clinically significant abnormality while on study therapy
N = total number of patients with at least one observation of the given lab test while on study therapy ULN = upper limit of normal LLN = lower limit of normal |
|||
|
|
|||
| Criteria * | Voriconazole | Amphotericin B followed by Fluconazole | |
| n/N (%) | n/N (%) | ||
| T. Bilirubin | >1.5x ULN | 50/261 (19.2) | 31/115 (27.0) |
| AST | >3.0x ULN | 40/261 (15.3) | 16/116 (13.8) |
| ALT | >3.0x ULN | 22/261 (8.4) | 15/116 (12.9) |
| Alk phos | >3.0x ULN | 59/261 (22.6) | 26/115 (22.6) |
| Creatinine | >1.3x ULN | 39/260 (15.0) | 32/118 (27.1) |
| Potassium | <0.9x LLN | 43/258 (16.7) | 35/118 (29.7) |
6.4 Postmarketing Experience
The following adverse reactions have been identified during post approval use of voriconazole. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skeletal: fluorosis and periostitis have been reported during long-term voriconazole therapy [see Warnings and Precautions (5.14)].
7 DRUG INTERACTIONS
|
|
||
| Drug/Drug Class
(Mechanism of Interaction by the Drug) | Voriconazole Plasma Exposure
(C max and AUC τ after 200 mg q12h) | Recommendations for Voriconazole
Dosage Adjustment/Comments |
| Rifampin
* and Rifabutin
*
(CYP450 Induction) | Significantly Reduced | Contraindicated |
| Efavirenz
†
(CYP450 Induction) | Significantly Reduced | When voriconazole is coadministered with efavirenz, voriconazole oral maintenance dose should be increased to 400 mg q12h and efavirenz should be decreased to 300 mg q24h |
| High-dose Ritonavir (400 mg q12h)
†
(CYP450 Induction) | Significantly Reduced | Contraindicated |
| Low-dose Ritonavir (100 mg q12h)
†
(CYP450 Induction) | Reduced | Coadministration of voriconazole and low-dose ritonavir (100 mg q12h) should be avoided, unless an assessment of the benefit/risk to the patient justifies the use of voriconazole |
| Carbamazepine
(CYP450 Induction) | Not Studied In Vivo or In Vitro, but Likely to Result in Significant Reduction | Contraindicated |
| Long Acting Barbiturates
(CYP450 Induction) | Not Studied In Vivo or In Vitro, but Likely to Result in Significant Reduction | Contraindicated |
| Phenytoin
*
(CYP450 Induction) | Significantly Reduced | Increase voriconazole maintenance dose from 4 mg/kg to 5 mg/kg IV q12h or from 200 mg to 400 mg orally q12h (100 mg to 200 mg orally q12h in patients weighing less than 40 kg) |
| St. John’s Wort
(CYP450 inducer; P-gp inducer) | Significantly Reduced | Contraindicated |
| Oral Contraceptives
†
containing ethinyl estradiol and norethindrone (CYP2C19 Inhibition) | Increased | Monitoring for adverse events and toxicity related to voriconazole is recommended when coadministered with oral contraceptives |
| Fluconazole
†(CYP2C9, CYP2C19 and
CYP3A4 Inhibition) | Significantly Increased | Avoid concomitant administration of voriconazole and fluconazole. Monitoring for adverse events and toxicity related to voriconazole is started within 24 h after the last dose of fluconazole. |
| Other HIV Protease Inhibitors
(CYP3A4 Inhibition) | In Vivo Studies Showed No Significant Effects of Indinavir on Voriconazole Exposure | No dosage adjustment in the voriconazole dosage needed when coadministered with indinavir |
| In Vivo Studies Demonstrated Potential for Inhibition of Voriconazole Metabolism (Increased Plasma Exposure) | Frequent monitoring for adverse events and toxicity related to voriconazole when coadministered with other HIV protease inhibitors | |
| Other NNRTIs
‡
(CYP3A4 Inhibition or CYP450 Induction) | In Vivo Studies Demonstrated Potential for Inhibition of Voriconazole Metabolism by Delavirdine and Other NNRTIs (Increased Plasma Exposure) | Frequent monitoring for adverse events and toxicity related to voriconazole |
| A Voriconazole-Efavirenz Drug Interaction Study Demonstrated the Potential for the Metabolism of Voriconazole to be Induced by Efavirenz and Other NNRTIs (Decreased Plasma Exposure) | Careful assessment of voriconazole effectiveness | |
|
|
||
| Drug/Drug Class
(Mechanism of Interaction by Voriconazole) | Drug Plasma Exposure
(C max and AUC τ) | Recommendations for Drug Dosage
Adjustment/Comments |
| Sirolimus
*
(CYP3A4 Inhibition) | Significantly Increased | Contraindicated |
| Rifabutin
*
(CYP3A4 Inhibition) | Significantly Increased | Contraindicated |
| Efavirenz
†
(CYP3A4 Inhibition) | Significantly Increased | When voriconazole is coadministered with efavirenz, voriconazole oral maintenance dose should be increased to 400 mg q12h and efavirenz should be decreased to 300 mg q24h |
| High-dose Ritonavir (400 mg q12h)
†
(CYP3A4 Inhibition) | No Significant Effect of Voriconazole on Ritonavir C max or AUC τ | Contraindicated because of significant reduction of voriconazole C max and AUC τ |
| Low-dose Ritonavir (100 mg q12h) † | Slight Decrease in Ritonavir C max or AUC τ | Coadministration of voriconazole and low-dose ritonavir (100 mg q12h) should be avoided (due to the reduction in voriconazole C max and AUC τ) unless an assessment of the benefit/risk to the patient justifies the use of voriconazole |
| Terfenadine, Astemizole, Cisapride,
Pimozide, Quinidine (CYP3A4 Inhibition) | Not Studied In Vivo or In Vitro, but Drug Plasma Exposure Likely to be Increased | Contraindicated because of potential for QT prolongation and rare occurrence of torsade de pointes |
| Ergot Alkaloids
(CYP450 Inhibition) | Not Studied In Vivo or In Vitro, but Drug Plasma Exposure Likely to be Increased | Contraindicated |
| Cyclosporine
*
(CYP3A4 Inhibition) | AUC τ Significantly Increased; No Significant Effect on C max | When initiating therapy with VORICONAZOLE in patients already receiving cyclosporine, reduce the cyclosporine dose to one-half of the starting dose and follow with frequent monitoring of cyclosporine blood levels. Increased cyclosporine levels have been associated with nephrotoxicity. When VORICONAZOLE is discontinued, cyclosporine concentrations must be frequently monitored and the dose increased as necessary. |
| Methadone
‡
(CYP3A4 Inhibition) | Increased | Increased plasma concentrations of methadone have been associated with toxicity including QT prolongation. Frequent monitoring for adverse events and toxicity related to methadone is recommended during coadministration. Dose reduction of methadone may be needed |
| Fentanyl (CYP3A4 Inhibition) | Increased | Reduction in the dose of fentanyl and other long-acting opiates metabolized by CYP3A4 should be considered when coadministered with Voriconazole. Extended and frequent monitoring for opiateassociated adverse events may be necessary [see Drug Interactions (7)] |
| Alfentanil (CYP3A4 Inhibition) | Significantly Increased | Reduction in the dose of alfentanil and other opiates metabolized by CYP3A4 (e.g., sufentanil) should be considered when coadministered with VORICONAZOLE. A longer period for monitoring respiratory and other opiate-associated adverse events may be necessary [see Drug Interactions (7)]. |
| Oxycodone (CYP3A4 Inhibition) | Significantly Increased | Reduction in the dose of oxycodone and other long-acting opiates metabolized by CYP3A4 should be considered when coadministered with voriconazole. Extended and frequent monitoring for opiateassociated adverse events may be necessary [see Drug Interactions (7)]. |
| NSAID
s§including. ibuprofen and diclofenac
(CYP2C9 Inhibition) | Increased | Frequent monitoring for adverse events and toxicity related to NSAIDs. Dose reduction of NSAIDs may be needed [see Drug Interactions (7)]. |
| Tacrolimus
*
(CYP3A4 Inhibition) | Significantly Increased | When initiating therapy with VORICONAZOLE in patients already receiving tacrolimus, reduce the tacrolimus dose to one-third of the starting dose and follow with frequent monitoring of tacrolimus blood levels. Increased tacrolimus levels have been associated with nephrotoxicity. When VORICONAZOLE is discontinued, tacrolimus concentrations must be frequently monitored and the dose increased as necessary. |
| Phenytoin
*
(CYP2C9 Inhibition) | Significantly Increased | Frequent monitoring of phenytoin plasma concentrations and frequent monitoring of adverse effects related to phenytoin. |
| Oral Contraceptives containing ethinylestradiol and norethindrone (CYP3A4 Inhibition) † | Increased | Monitoring for adverse events related to oral contraceptives is recommended during coadministration. |
| Warfarin
*
(CYP2C9 Inhibition) | Prothrombin Time Significantly Increased | Monitor PT or other suitable anticoagulation tests. Adjustment of warfarin dosage may be needed. |
| Omeprazole
*
(CYP2C19/3A4 Inhibition) | Significantly Increased | When initiating therapy with VORICONAZOLE in patients already receiving omeprazole doses of 40 mg or greater, reduce the omeprazole dose by one-half. The metabolism of other proton pump inhibitors that are CYP2C19 substrates may also be inhibited by voriconazole and may result in increased plasma concentrations of other proton pump inhibitors. |
| Other HIV Protease Inhibitors
(CYP3A4 Inhibition) | In Vivo Studies Showed No Significant Effects on Indinavir Exposure | No dosage adjustment for indinavir when coadministered with VORICONAZOLE |
| In Vitro Studies Demonstrated Potential for Voriconazole to Inhibit Metabolism (Increased Plasma Exposure) | Frequent monitoring for adverse events and toxicity related to other HIV protease inhibitors | |
| Other NNRTIs
¶
(CYP3A4 Inhibition) | A Voriconazole-Efavirenz Drug Interaction Study Demonstrated the Potential for Voriconazole to Inhibit Metabolism of Other NNRTIs (Increased Plasma Exposure) | Frequent monitoring for adverse events and toxicity related to NNRTI |
| Benzodiazepines
(CYP3A4 Inhibition) | In Vitro Studies Demonstrated Potential for Voriconazole to Inhibit Metabolism (Increased Plasma Exposure) | Frequent monitoring for adverse events and toxicity (i.e., prolonged sedation) related to benzodiazepines metabolized by CYP3A4 (e.g., midazolam, triazolam, alprazolam). Adjustment of benzodiazepine dosage may be needed. |
| HMG-CoA Reductase Inhibitors (Statins)
(CYP3A4 Inhibition) | In Vitro Studies Demonstrated Potential for Voriconazole to Inhibit Metabolism (Increased Plasma Exposure) | Frequent monitoring for adverse events and toxicity related to statins. Increased statin concentrations in plasma have been associated with rhabdomyolysis. Adjustment of the statin dosage may be needed. |
| Dihydropyridine Calcium Channel Blockers
(CYP3A4 Inhibition) | In Vitro Studies Demonstrated Potential for Voriconazole to Inhibit Metabolism (Increased Plasma Exposure) | Frequent monitoring for adverse events and toxicity related to calcium channel blockers. Adjustment of calcium channel blocker dosage may be needed. |
| Sulfonylurea Oral Hypoglycemics
(CYP2C9 Inhibition) | Not Studied In Vivo or In Vitro but Drug Plasma Exposure Likely to be Increased | Frequent monitoring of blood glucose and for signs and symptoms of hypoglycemia. Adjustment of oral hypoglycemic drug dosage may be needed. |
| Vinca Alkaloids
(CYP3A4 Inhibition) | Not Studied In Vivo or In Vitro but Drug Plasma Exposure Likely to be Increased | Frequent monitoring for adverse events and toxicity (i.e., neurotoxicity) related to vinca alkaloids. Adjustment of vinca alkaloid dosage may be needed. |
| Everolimus
(CYP3A4 Inhibition) | Not Studied In Vivo or In Vivo or In Vitro but Drug Plasma Exposure Likely to be Increased | Concomitant administration of voriconazole and everolimus is not recommended. |
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy Pregnancy
Category D
Voriconazole can cause fetal harm when administered to a pregnant woman and should not be taken in pregnancy except in patients where the benefit to the mother clearly outweighs the potential risk to the fetus. There are no adequate and well-controlled studies in pregnant women
If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patients should be informed of the potential hazard to the fetus [ see Warnings and Precautions (5.4)].
Animal Data
Voriconazole was teratogenic in rats (cleft palates, hydronephrosis/hydroureter) from 10 mg/kg (0.3 times the recommended maintenance dose (RMD) on a mg/m 2 basis) and embryotoxic in rabbits at 100 mg/kg (6 times the RMD). Other effects in rats included reduced ossification of sacral and caudal vertebrae, skull, pubic and hyoid bone, supernumerary ribs, anomalies of the sternebrae and dilatation of the ureter/renal pelvis. Plasma estradiol in pregnant rats was reduced at all dose levels. Voriconazole treatment in rats produced increased gestational length and dystocia, which were associated with increased perinatal pup mortality at the 10 mg/kg dose. The effects seen in rabbits were an increased embryomortality, reduced fetal weight and increased incidences of skeletal variations, cervical ribs and extrasternebral ossification sites.
8.3 Nursing Mothers
It is not known whether voriconazole is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from VORICONAZOLE, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Uses
Safety and effectiveness in pediatric patients below the age of 12 years have not been established.
A total of 22 patients aged 12 to 18 years with invasive aspergillosis were included in the therapeutic studies. Twelve out of 22 (55%) patients had successful response after treatment with a maintenance dose of voriconazole 4 mg/kg q12h.
Sparse plasma sampling for pharmacokinetics in adolescents was conducted in the therapeutic studies [see Clinical Pharmacology (12.3)].
There have been postmarketing reports of pancreatitis in pediatric patients.
8.5 Geriatric Use
In multiple dose therapeutic trials of voriconazole, 9.2% of patients were ≥65 years of age and 1.8% of patients were ≥75 years of age. In a study in healthy subjects, the systemic exposure (AUC) and peak plasma concentrations (Cmax) were increased in elderly males compared to young males. Pharmacokinetic data obtained from 552 patients from 10 voriconazole therapeutic trials showed that voriconazole plasma concentrations in the elderly patients were approximately 80% to 90% higher than those in younger patients after either IV or oral administration. However, the overall safety profile of the elderly patients was similar to that of the young so no dosage adjustment is recommended [see Clinical Pharmacology (12.3)].
8.6 Women of Childbearing Potential
Women of childbearing potential should use effective contraception during treatment. The coadministration of voriconazole with the oral contraceptive, Ortho-Novum® (35 mcg ethinyl estradiol and 1 mg norethindrone), results in an interaction between these two drugs, but is unlikely to reduce the contraceptive effect. Monitoring for adverse events associated with oral contraceptives and voriconazole is recommended [see Drug Interactions (7), and Clinical Pharmacology (12.3)].
10 OVERDOSAGE
In clinical trials, there were three cases of accidental overdose. All occurred in pediatric patients who received up to five times the recommended intravenous dose of voriconazole. A single adverse event of photophobia of 10 minutes duration was reported.
There is no known antidote to voriconazole.
Voriconazole is hemodialyzed with clearance of 121 mL/min. The intravenous vehicle, SBECD, is hemodialyzed with clearance of 55 mL/min. In an overdose, hemodialysis may assist in the removal of voriconazole and SBECD from the body.
The minimum lethal oral dose in mice and rats was 300 mg/kg (equivalent to 4 and 7 times the recommended maintenance dose (RMD), based on body surface area). At this dose, clinical signs observed in both mice and rats included salivation, mydriasis, titubation (loss of balance while moving), depressed behavior, prostration, partially closed eyes, and dyspnea. Other signs in mice were convulsions, corneal opacification and swollen abdomen.
11 DESCRIPTION
VORICONAZOLE ® (voriconazole), an azole antifungal agent is available as a film-coated tablets for oral administration. The structural formula is:
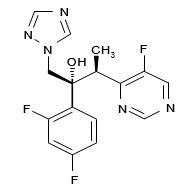
Voriconazole is designated chemically as (2R,3S)-2-(2, 4-difluorophenyl)-3-(5-fluoro-4-pyrimidinyl)-1-(1 H-1,2,4- triazol-1-yl)-2-butanol with an empirical formula of C 16H 14F 3N 5O and a molecular weight of 349.31.
Voriconazole drug substance is a white to light-colored powder.
VORICONAZOLE Tablets contain 50 mg or 200 mg of voriconazole. The inactive ingredients include lactose monohydrate, pregelatinized starch, croscarmellose sodium, povidone, magnesium stearate and a coating containing hypromellose, titanium dioxide, lactose monohydrate and triacetin.
12 CLINICALPHARMACOLOGY
12.3 Pharmacokinetics
General Pharmacokinetic Characteristics
The pharmacokinetics of voriconazole have been characterized in healthy subjects, special populations and patients.
During oral administration of 200 mg or 300 mg twice daily for 14 days in patients at risk of aspergillosis (mainly patients with malignant neoplasms of lymphatic or hematopoietic tissue), the observed pharmacokinetic characteristics were similar to those observed in healthy subjects.
The pharmacokinetics of voriconazole are non-linear due to saturation of its metabolism. The interindividual variability of voriconazole pharmacokinetics is high. Greater than proportional increase in exposure is observed with increasing dose. It is estimated that, on average, increasing the oral dose from 200 mg q12h to 300 mg q12h leads to an approximately 2.5-fold increase in exposure (AUC 1); similarly, increasing the intravenous dose from 3 mg/kg q12h to 4 mg/kg q12h produces an approximately 2.5-fold increase in exposure (Table 9).
|
Note: Parameters were estimated based on non-compartmental analysis from 5 pharmacokinetic studies.
AUC 12 = area under the curve over 12 hour dosing interval, C max = maximum plasma concentration, C min = minimum plasma concentration. CV = coefficient of variation. |
||||||
| 6 mg/kg IV
(loading dose) | 3 mg/kg
IV q12h | 4 mg/kg
IV q12h | 400 mg Oral
(loading dose) | 200 mg
Oral q12h | 300 mg
Oral q12h |
|
|---|---|---|---|---|---|---|
| N | 35 | 23 | 40 | 17 | 48 | 16 |
| AUC 12 (μgh/mL) | 13.9 (32) | 13.7 (53) | 33.9 (54) | 9.31 (38) | 12.4 (78) | 34.0 (53) |
| C max (μg/mL) | 3.13 (20) | 3.03 (25) | 4.77 (36) | 2.30 (19) | 2.31 (48) | 4.74 (35) |
| C min (μg/mL) | -- | 0.46 (97) | 1.73 (74) | -- | 0.46 (120) | 1.63 (79) |
Sparse plasma sampling for pharmacokinetics was conducted in the therapeutic studies in patients aged 12-18 years. In 11 adolescent patients who received a mean voriconazole maintenance dose of 4 mg/kg IV, the median of the calculated mean plasma concentrations was 1.60 μg/mL (inter-quartile range 0.28 to 2.73 μg/mL). In 17 adolescent patients for whom mean plasma concentrations were calculated following a mean oral maintenance dose of 200 mg q12h, the median of the calculated mean plasma concentrations was 1.16 μg/mL (inter-quartile range 0.85 to 2.14 μg/mL).
Absorption–The pharmacokinetic properties of voriconazole are similar following administration by the intravenous and oral routes. Based on a population pharmacokinetic analysis of pooled data in healthy subjects (N=207), the oral bioavailability of voriconazole is estimated to be 96% (CV 13%). Bioequivalence was established between the 200 mg tablet.when administered as a 400 mg q12h loading dose followed by a 200 mg q12h maintenance dose.
Maximum plasma concentrations (C max) are achieved 1-2 hours after dosing. When multiple doses of voriconazole are administered with high-fat meals, the mean C max and AUC T are reduced by 34% and 24%, respectively when administered as a tablet and by 58% and 37% respectively.
In healthy subjects, the absorption of voriconazole is not affected by coadministration of oral ranitidine, cimetidine, or omeprazole, drugs that are known to increase gastric pH.
Distribution–The volume of distribution at steady state for voriconazole is estimated to be 4.6 L/kg, suggesting extensive distribution into tissues. Plasma protein binding is estimated to be 58% and was shown to be independent of plasma concentrations achieved following single and multiple oral doses of 200 mg or 300 mg (approximate range: 0.9-15 μg/mL). Varying degrees of hepatic and renal insufficiency do not affect the protein binding of voriconazole.
Metabolism–In vitro studies showed that voriconazole is metabolized by the human hepatic cytochrome P450 enzymes, CYP2C19, CYP2C9 and CYP3A4 [ see Drug Interactions (7)].
In vivo studies indicated that CYP2C19 is significantly involved in the metabolism of voriconazole. This enzyme exhibits genetic polymorphism. For example, 15-20% of Asian populations may be expected to be poor metabolizers. For Caucasians and Blacks, the prevalence of poor metabolizers is 3-5%. Studies conducted in Caucasian and Japanese healthy subjects have shown that poor metabolizers have, on average, 4-fold higher voriconazole exposure (AUC T) than their homozygous extensive metabolizer counterparts. Subjects who are heterozygous extensive metabolizers have, on average, 2-fold higher voriconazole exposure than their homozygous extensive metabolizer counterparts.
The major metabolite of voriconazole is the N-oxide, which accounts for 72% of the circulating radiolabelled metabolites in plasma. Since this metabolite has minimal antifungal activity, it does not contribute to the overall efficacy of voriconazole.
Excretion–Voriconazole is eliminated via hepatic metabolism with less than 2% of the dose excreted unchanged in the urine. After administration of a single radiolabelled dose of either oral or IV voriconazole, preceded by multiple oral or IV dosing, approximately 80% to 83% of the radioactivity is recovered in the urine. The majority (>94%) of the total radioactivity is excreted in the first 96 hours after both oral and intravenous dosing.
As a result of non-linear pharmacokinetics, the terminal half-life of voriconazole is dose dependent and therefore not useful in predicting the accumulation or elimination of voriconazole.
Pharmacokinetic-Pharmacodynamic Relationships
Clinical Efficacy and Safety–In 10 clinical trials, the median values for the average and maximum voriconazole plasma concentrations in individual patients across these studies (N=1121) was 2.51 µg/mL (inter-quartile range 1.21 to 4.44 µg/mL) and 3.79 µg/mL (inter-quartile range 2.06 to 6.31 µg/mL), respectively. A pharmacokinetic pharmacodynamic analysis of patient data from 6 of these 10 clinical trials (N=280) could not detect a positive association between mean, maximum or minimum plasma voriconazole concentration and efficacy. However, pharmacokinetic/pharmacodynamic analyses of the data from all 10 clinical trials identified positive associations between plasma voriconazole concentrations and rate of both liver function test abnormalities and visual disturbances [ see Adverse Reactions (6)].
Electrocardiogram–A placebo-controlled, randomized, crossover study to evaluate the effect on the QT interval of healthy male and female subjects was conducted with three single oral doses of voriconazole and ketoconazole. Serial ECGs and plasma samples were obtained at specified intervals over a 24-hour post dose observation period. The placebo-adjusted mean maximum increases in QTc from baseline after 800, 1200 and 1600 mg of voriconazole and after ketoconazole 800 mg were all <10 msec. Females exhibited a greater increase in QTc than males, although all mean changes were <10 msec. Age was not found to affect the magnitude of increase in QTc. No subject in any group had an increase in QTc of ≥60 msec from baseline. No subject experienced an interval exceeding the potentially clinically relevant threshold of 500 msec. However, the QT effect of voriconazole combined with drugs known to prolong the QT interval is unknown [ see Contraindications (4) andDrug Interactions (7)].
Pharmacokinetics in Special Populations
Gender–In a multiple oral dose study, the mean C max and AUC T for healthy young females were 83% and 113% higher, respectively, than in healthy young males (18-45 years), after tablet dosing. In the same study, no significant differences in the mean C max and AUC T were observed between healthy elderly males and healthy elderly females (>65 years). The steady state trough voriconazole concentrations (C min) seen in females were 100% and 91% higher than in males receiving the tablet.
In the clinical program, no dosage adjustment was made on the basis of gender. The safety profile and plasma concentrations observed in male and female subjects were similar. Therefore, no dosage adjustment based on gender is necessary.
Geriatric–In an oral multiple dose study the mean C max and AUC T in healthy elderly males (≥65 years) were 61% and 86% higher, respectively, than in young males (18-45 years). No significant differences in the mean C max and AUC T were observed between healthy elderly females (≥65 years) and healthy young females (18-45 years).
In the clinical program, no dosage adjustment was made on the basis of age. An analysis of pharmacokinetic data obtained from 552 patients from 10 voriconazole clinical trials showed that the median voriconazole plasma concentrations in the elderly patients (>65 years) were approximately 80% to 90% higher than those in the younger patients (≤65 years) after either IV or oral administration. However, the safety profile of voriconazole in young and elderly subjects was similar and, therefore, no dosage adjustment is necessary for the elderly [see Use in Special Populations (8.5)].
Pediatric–A population pharmacokinetic analysis was conducted on pooled data from 35 immunocompromised pediatric patients aged 2 to <12 years old who were included in two pharmacokinetic studies of intravenous voriconazole (single dose and multiple dose). Twenty-four of these patients received multiple intravenous maintenance doses of 3 mg/kg and 4 mg/kg. A comparison of the pediatric and adult population pharmacokinetic data revealed that the predicted average steady state plasma concentrations were similar at the maintenance dose of 4 mg/kg every 12 hours in children and 3 mg/kg every 12 hours in adults (medians of 1.19 μg/mL and 1.16 μg/mL in children and adults, respectively) [see Use in Specific Populations (8.4)].
Hepatic Impairment–After a single oral dose (200 mg) of voriconazole in 8 patients with mild (Child-Pugh Class A) and 4 patients with moderate (Child-Pugh Class B) hepatic insufficiency, the mean systemic exposure (AUC) was 3.2-fold higher than in age and weight matched controls with normal hepatic function. There was no difference in mean peak plasma concentrations (C max) between the groups. When only the patients with mild (Child-Pugh Class A) hepatic insufficiency were compared to controls, there was still a 2.3-fold increase in the mean AUC in the group with hepatic insufficiency compared to controls.
In an oral multiple dose study, AUCτ was similar in 6 subjects with moderate hepatic impairment (Child-Pugh Class B) given a lower maintenance dose of 100 mg twice daily compared to 6 subjects with normal hepatic function given the standard 200 mg twice daily maintenance dose. The mean peak plasma concentrations (C max) were 20% lower in the hepatically impaired group.
It is recommended that the standard loading dose regimens be used but that the maintenance dose be halved in patients with mild to moderate hepatic cirrhosis (Child-Pugh Class A and B) receiving voriconazole. No pharmacokinetic data are available for patients with severe hepatic cirrhosis (Child-Pugh Class C) [see Dosage and Administration (2.7)].
Renal Impairment–In a single oral dose (200 mg) study in 24 subjects with normal renal function and mild to severe renal impairment, systemic exposure (AUC) and peak plasma concentration (C max) of voriconazole were not significantly affected by renal impairment. Therefore, no adjustment is necessary for oral dosing in patients with mild to severe renal impairment.
In a multiple dose study of IV voriconazole (6 mg/kg IV loading dose x 2, then 3 mg/kg IV x 5.5 days) in 7 patients with moderate renal dysfunction (creatinine clearance 30-50 mL/min), the systemic exposure (AUC) and peak plasma concentrations (C max) were not significantly different from those in 6 subjects with normal renal function.
However, in patients with moderate renal dysfunction (creatinine clearance 30-50 mL/min), accumulation of the intravenous vehicle, SBECD, occurs. The mean systemic exposure (AUC) and peak plasma concentrations (C max) of SBECD were increased 4-fold and almost 50%, respectively, in the moderately impaired group compared to the normal control group.
Intravenous voriconazole should be avoided in patients with moderate or severe renal impairment (creatinine clearance <50 mL/min), unless an assessment of the benefit/risk to the patient justifies the use of intravenous voriconazole [see Dosage and Administration (2.8)].
A pharmacokinetic study in subjects with renal failure undergoing hemodialysis showed that voriconazole is dialyzed with clearance of 121 mL/min. The intravenous vehicle, SBECD, is hemodialyzed with clearance of 55 mL/min. A 4-hour hemodialysis session does not remove a sufficient amount of voriconazole to warrant dose adjustment.
Drug Interactions
Effects of Other Drugs on Voriconazole
Voriconazole is metabolized by the human hepatic cytochrome P450 enzymes CYP2C19, CYP2C9, and CYP3A4. Results of in vitro metabolism studies indicate that the affinity of voriconazole is highest for CYP2C19, followed by CYP2C9, and is appreciably lower for CYP3A4. Inhibitors or inducers of these three enzymes may increase or decrease voriconazole systemic exposure (plasma concentrations), respectively.
The systemic exposure to voriconazole is significantly reduced or is expected to be reduced by the concomitant administration of the following agents and their use is contraindicated:
Rifampin (potent CYP450 inducer) –Rifampin (600 mg once daily) decreased the steady state C max and AUC T of voriconazole (200 mg q12h x 7 days) by an average of 93% and 96%, respectively, in healthy subjects. Doubling the dose of voriconazole to 400 mg q12h does not restore adequate exposure to voriconazole during coadministration with rifampin. Coadministration of voriconazole and rifampin is contraindicated [see Contraindications (4), and Warnings and Precautions (5.1)].
Ritonavir (potent CYP450 inducer; CYP3A4 inhibitor and substrate) )–The effect of the coadministration of voriconazole and ritonavir (400 mg and 100 mg) was investigated in two separate studies. High-dose ritonavir (400 mg q12h for 9 days) decreased the steady state C max and AUC T of oral voriconazole (400 mg q12h for 1 day, then 200 mg q12h for 8 days) by an average of 66% and 82%, respectively, in healthy subjects. Low-dose ritonavir (100 mg q12h for 9 days) decreased the steady state C max and AUC T of oral voriconazole (400 mg q12h for 1 day, then 200 mg q12h for 8 days) by an average of 24% and 39%, respectively, in healthy subjects. Although repeat oral administration of voriconazole did not have a significant effect on steady state C max and AUC T of high-dose ritonavir in healthy subjects, steady state C max and AUC T of low-dose ritonavir decreased slightly by 24% and 14% respectively, when administered concomitantly with oral voriconazole in healthy subjects. Coadministration of voriconazole and high-dose ritonavir (400 mg q12h) is contraindicated. Coadministration of voriconazole and low-dose ritonavir (100 mg q12h) should be avoided, unless an assessment of the benefit/risk to the patient justifies the use of voriconazole [see Contraindications (4), and Warnings and Precautions (5.1)].
St. John’s Wort (CYP450 inducer; P-gp inducer) –In an independent published study in healthy volunteers who were given multiple oral doses of St. John’s Wort (300 mg LI 160 extract three times daily for 15 days) followed by a single 400 mg oral dose of voriconazole, a 59% decrease in mean voriconazole AUC 0-∞ was observed. In contrast, coadministration of single oral doses of St. John’s Wort and voriconazole had no appreciable effect on voriconazole AUC 0-∞. Because long-term use of St. John’s Wort could lead to reduced voriconazole exposure, concomitant use of voriconazole with St. John’s Wort is contraindicated [ see Contraindications (4)].
Carbamazepine and long-acting barbiturates (potent CYP450 inducers) –Although not studied in vitro or in vivo, carbamazepine and long-acting barbiturates (e.g., phenobarbital, mephobarbital) are likely to significantly decrease plasma voriconazole concentrations. Coadministration of voriconazole with carbamazepine or long-acting barbiturates is contraindicated [ see Contraindications (4), and Warnings and Precautions (5.1)].
Significant drug interactions that may require voriconazole dosage adjustment, or frequent monitoring of voriconazole-related adverse events/toxicity:
Fluconazole (CYP2C9, CYP2C19 and CYP3A4 inhibitor): Concurrent administration of oral voriconazole (400 mg q12h for 1 day, then 200 mg q12h for 2.5 days) and oral fluconazole (400 mg on day 1, then 200 mg Q24h for 4 days) to 6 healthy male subjects resulted in an increase in Cmax and AUCτ of voriconazole by an average of 57% (90% CI: 20%, 107%) and 79% (90% CI: 40%, 128%), respectively. In a follow-on clinical study involving 8 healthy male subjects, reduced dosing and/or frequency of voriconazole and fluconazole did not eliminate or diminish this effect. Concomitant administration of voriconazole and fluconazole at any dose is not recommended. Close monitoring for adverse events related to voriconazole is recommended if voriconazole is used sequentially after fluconazole, especially within 24 hours of the last dose of fluconazole [ see Warnings and Precautions (5.1)].
Minor or no significant pharmacokinetic interactions that do not require dosage adjustment:
Cimetidine (non-specific CYP450 inhibitor and increases gastric pH) –Cimetidine (400 mg q12h x 8 days) increased voriconazole steady state C max and AUC T by an average of 18% (90% CI: 6%, 32%) and 23% (90% CI: 13%, 33%), respectively, following oral doses of 200 mg q12h x 7 days to healthy subjects.
Ranitidine (increases gastric pH) )–Ranitidine (150 mg q12h) had no significant effect on voriconazole C max and AUC T following oral doses of 200 mg q12h x 7 days to healthy subjects.
Macrolide antibiotics–Coadministration of erythromycin (CYP3A4 inhibitor; 1g q12h for 7 days) or azithromycin (500 mg qd for 3 days) with voriconazole 200 mg q12h for 14 days had no significant effect on voriconazole steady state C max and AUC T in healthy subjects. The effects of voriconazole on the pharmacokinetics of either erythromycin or azithromycin are not known.
Effects of Voriconazole on Other Drugs
In vitro studies with human hepatic microsomes show that voriconazole inhibits the metabolic activity of the cytochrome P450 enzymes CYP2C19, CYP2C9, and CYP3A4. In these studies, the inhibition potency of voriconazole for CYP3A4 metabolic activity was significantly less than that of two other azoles, ketoconazole and itraconazole. In vitro studies also show that the major metabolite of voriconazole, voriconazole N-oxide, inhibits the metabolic activity of CYP2C9 and CYP3A4 to a greater extent than that of CYP2C19. Therefore, there is potential for voriconazole and its major metabolite to increase the systemic exposure (plasma concentrations) of other drugs metabolized by these CYP450 enzymes.
The systemic exposure of the following drugs is significantly increased or is expected to be significantly increased by coadministration of voriconazole and their use is contraindicated:
Sirolimus (CYP3A4 substrate) (CYP3A4 substrate)–Repeat dose administration of oral voriconazole (400 mg q12h for 1 day, then 200 mg q12h for 8 days) increased the C max and AUC of sirolimus (2 mg single dose) an average of 7-fold (90% CI: 5.7, 7.5) and 11-fold (90% CI: 9.9, 12.6), respectively, in healthy male subjects. Coadministration of voriconazole and sirolimus is contraindicated [ see Contraindications (4), and Warnings and Precautions (5.1)].
Terfenadine, astemizole, cisapride, pimozide and quinidine (CYP3A4 substrates)–Although not studied in vitro or in vivo, concomitant administration of voriconazole with terfenadine, astemizole, cisapride, pimozide or quinidine may result in inhibition of the metabolism of these drugs. Increased plasma concentrations of these drugs can lead to QT prolongation and rare occurrences of torsade de pointes. Coadministration of voriconazole and terfenadine, astemizole, cisapride, pimozide and quinidine is contraindicated[ see Contraindications (4), and Warnings and Precautions (5.1)].
Ergot alkaloids–Although not studied in vitro or in vivo, voriconazole may increase the plasma concentration of ergot alkaloids (ergotamine and dihydroergotamine) and lead to ergotism. . Coadministration of voriconazole with ergot alkaloids is contraindicated [ see Contraindications (4), and Warnings and Precautions (5.1)].
Everolimus (CYP3A4 substrate, P-gp substrate) -Although not studied in vitro or in vivo, voriconazole may increase plasma concentrations of everolimus, which could potentially lead to exacerbation of everolimus toxicity. Currently there are insufficient data to allow dosing recommendations in this situation. Therefore, co-administration of voriconazole with everolimus is not recommended [ see Drug Interactions (7)].
Coadministration of voriconazole with the following agents results in increased exposure or is expected to result in increased exposure to these drugs. Therefore, careful monitoring and/or dosage adjustment of these drugs is needed:
Alfentanil (CYP3A4 substrate)–Coadministration of multiple doses of oral voriconazole (400 mg q12h on day 1, 200 mg q12h on day 2) with a single 20 mcg/kg intravenous dose of alfentanil with concomitant naloxone resulted in a 6-fold increase in mean alfentanil AUC 0-∞ and a 4-fold prolongation of mean alfentanil elimination half-life, compared to when alfentanil was given alone. An increase in the incidence of delayed and persistent alfentanil associated nausea and vomiting during co-administration of voriconazole and alfentanil was also observed. Reduction in the dose of alfentanil or other opiates that are also metabolized by CYP3A4 (e.g., sufentanil), and extended close monitoring of patients for respiratory and other opiate-associated adverse events, may be necessary when any of these opiates is coadministered with voriconazole [ see Warnings and Precautions (5.1)].
Fentanyl (CYP3A4 substrate): In an independent published study, concomitant use of voriconazole (400 mg q12h on Day 1, then 200 mg q12h on Day 2) with a single intravenous dose of fentanyl (5 µg/kg) resulted in an increase in the mean AUC 0-∞ of fentanyl by 1.4-fold (range 0.81- to 2.04-fold). When voriconazole is co-administered with fentanyl IV, oral or transdermal dosage forms, extended and frequent monitoring of patients for respiratory depression and other fentanyl-associated adverse events is recommended, and fentanyl dosage should be reduced if warranted [ see Warnings and Precautions (5.1)].
Oxycodone (CYP3A4 substrate): In an independent published study, coadministration of multiple doses of oral voriconazole (400 mg q12h, on Day 1 followed by five doses of 200 mg q12h on Days 2 to 4) with a single 10 mg oral dose of oxycodone on Day 3 resulted in an increase in the mean C max and AUC 0–∞ of oxycodone by 1.7-fold (range 1.4- to 2.2-fold) and 3.6-fold (range 2.7- to 5.6-fold), respectively. The mean elimination half-life of oxycodone was also increased by 2.0-fold (range 1.4- to 2.5-fold). Voriconazole also increased the visual effects (heterophoria and miosis) of oxycodone. A reduction in oxycodone dosage may be needed during voriconazole treatment to avoid opioid related adverse effects. Extended and frequent monitoring for adverse effects associated with oxycodone and other long-acting opiates metabolized by CYP3A4 is recommended [ see Warnings and Precautions (5.1)].
Cyclosporine (CYP3A4 substrate) –In stable renal transplant recipients receiving chronic cyclosporine therapy, concomitant administration of oral voriconazole (200 mg q12h for 8 days) increased cyclosporine C max and AUC T an average of 1.1 times (90% CI: 0.9, 1.41) and 1.7 times (90% CI: 1.5, 2.0), respectively, as compared to when cyclosporine was administered without voriconazole. When initiating therapy with voriconazole in patients already receiving cyclosporine, it is recommended that the cyclosporine dose be reduced to one-half of the original dose and followed with frequent monitoring of the cyclosporine blood levels. Increased cyclosporine levels have been associated with nephrotoxicity. When voriconazole is discontinued, cyclosporine levels should be frequently monitored and the dose increased as necessary [ see Warnings and Precautions (5.1)].
Methadone (CYP3A4, CYP2C19, CYP2C9 substrate)–Repeat dose administration of oral voriconazole (400 mg q12h for 1 day, then 200 mg q12h for 4 days) increased the C max and AUC T of pharmacologically active Rmethadone by 31% (90% CI: 22%, 40%) and 47% (90% CI: 38%, 57%), respectively, in subjects receiving a methadone maintenance dose (30-100 mg q24h). The C max and AUC of (S)-methadone increased by 65% (90% CI: 53%, 79%) and 103% (90% CI: 85%, 124%), respectively. Increased plasma concentrations of methadone have been associated with toxicity including QT prolongation. Frequent monitoring for adverse events and toxicity related to methadone is recommended during coadministration. Dose reduction of methadone may be needed [ see Warnings and Precautions (5.1)].
Tacrolimus (CYP3A4 substrate) –Repeat oral dose administration of voriconazole (400 mg q12h x 1 day, then 200 mg q12h x 6 days) increased tacrolimus (0.1 mg/kg single dose) C max and AUC T in healthy subjects by an average of 2-fold (90% CI: 1.9, 2.5) and 3-fold (90% CI: 2.7, 3.8), respectively. When initiating therapy with voriconazole in patients already receiving tacrolimus, it is recommended that the tacrolimus dose be reduced to one-third of the original dose and followed with frequent monitoring of the tacrolimus blood levels. Increased tacrolimus levels have been associated with nephrotoxicity. When voriconazole is discontinued, tacrolimus levels should be carefully monitored and the dose increased as necessary [ see Warnings and Precautions (5.1)].
Warfarin (CYP2C9 substrate) –Coadministration of voriconazole (300 mg q12h x 12 days) with warfarin (30 mg single dose) significantly increased maximum prothrombin time by approximately 2 times that of placebo in healthy subjects. Close monitoring of prothrombin time or other suitable anticoagulation tests is recommended if warfarin and voriconazole are coadministered and the warfarin dose adjusted accordingly [ see Warnings and Precautions (5.1)].
Oral Coumarin Anticoagulants (CYP2C9, CYP3A4 substrates) –Although not studied in vitro or in vivo, voriconazole may increase the plasma concentrations of coumarin anticoagulants and therefore may cause an increase in prothrombin time. If patients receiving coumarin preparations are treated simultaneously with voriconazole, the prothrombin time or other suitable anti-coagulation tests should be monitored at close intervals and the dosage of anticoagulants adjusted accordingly [see Warnings and Precautions (5.1)].
Statins (CYP3A4 substrates) –Although not studied clinically, voriconazole has been shown to inhibit lovastatin metabolism in vitro (human liver microsomes). Therefore, voriconazole is likely to increase the plasma concentrations of statins that are metabolized by CYP3A4. It is recommended that dose adjustment of the statin be considered during coadministration. Increased statin concentrations in plasma have been associated with rhabdomyolysis [see Warnings and Precautions (5.1)].
Benzodiazepines (CYP3A4 substrates) –Although not studied clinically, voriconazole has been shown to inhibit midazolam metabolism in vitro (human liver microsomes). Therefore, voriconazole is likely to increase the plasma concentrations of benzodiazepines that are metabolized by CYP3A4 (e.g., midazolam, triazolam, and alprazolam) and lead to a prolonged sedative effect. It is recommended that dose adjustment of the benzodiazepine be considered during coadministration [see Warnings and Precautions (5.1)].
Calcium Channel Blockers (CYP3A4 substrates) –Although not studied clinically, voriconazole has been shown to inhibit felodipine metabolism in vitro (human liver microsomes). Therefore, voriconazole may increase the plasma concentrations of calcium channel blockers that are metabolized by CYP3A4. Frequent monitoring for adverse events and toxicity related to calcium channel blockers is recommended during coadministration. Dose adjustment of the calcium channel blocker may be needed [ see Warnings and Precautions (5.1)].
Sulfonylureas (CYP2C9 substrates) –Although not studied in vitro or in vivo, voriconazole may increase plasma concentrations of sulfonylureas (e.g., tolbutamide, glipizide, and glyburide) and therefore cause hypoglycemia. Frequent monitoring of blood glucose and appropriate adjustment (i.e., reduction) of the sulfonylurea dosage is recommended during coadministration [ see Warnings and Precautions (5.1)].
Vinca Alkaloids (CYP3A4 substrates) –Although not studied in vitro or in vivo, voriconazole may increase the plasma concentrations of the vinca alkaloids (e.g., vincristine and vinblastine) and lead to neurotoxicity. Therefore, it is recommended that dose adjustment of the vinca alkaloid be considered [ see Warnings and Precautions (5.1)].
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs; CYP2C9 substrates): In two independent published studies, single doses of ibuprofen (400 mg) and diclofenac (50 mg) were coadministered with the last dose of voriconazole (400 mg q12h on Day 1, followed by 200 mg q12h on Day 2). Voriconazole increased the mean C max and AUC of the pharmacologically active isomer, S (+)-ibuprofen by 20% and 100%, respectively. Voriconazole increased the mean C max and AUC of diclofenac by 114% and 78%, respectively.
A reduction in ibuprofen and diclofenac dosage may be needed during concomitant administration with voriconazole. Patients receiving voriconazole concomitantly with other NSAIDs (e.g., celecoxib, naproxen, lornoxicam, meloxicam) that are also metabolized by CYP2C9 should be carefully monitored for NSAID-related adverse events and toxicity, and dosage reduction should be made if warranted [ see Warnings and Precautions (5.1)].
No significant pharmacokinetic interactions were observed when voriconazole was coadministered with the following agents. Therefore, no dosage adjustment for these agents is recommended:
Prednisolone (CYP3A4 substrate) –Voriconazole (200 mg q12h x 30 days) increased C max and AUC of prednisolone (60 mg single dose) by an average of 11% and 34%, respectively, in healthy subjects.
Digoxin (P-glycoprotein mediated transport) –Voriconazole (200 mg q12h x 12 days) had no significant effect on steady state C max and AUC T of digoxin (0.25 mg once daily for 10 days) in healthy subjects.
Mycophenolic acid (UDP-glucuronyl transferase substrate) –Voriconazole (200 mg q12h x 5 days) had no significant effect on the C max and AUC T of mycophenolic acid and its major metabolite, mycophenolic acid glucuronide after administration of a 1 g single oral dose of mycophenolate mofetil.
Two-Way Interactions
Concomitant use of the following agents with voriconazole is contraindicated:
Rifabutin (potent CYP450 inducer) –Rifabutin (300 mg once daily) decreased the C max and AUC T of voriconazole at 200 mg twice daily by an average of 67% (90% CI: 58%, 73%) and 79% (90% CI: 71%, 84%), respectively, in healthy subjects. During coadministration with rifabutin (300 mg once daily), the steady state C max and AUC T of voriconazole following an increased dose of 400 mg twice daily were on average approximately 2 times higher, compared with voriconazole alone at 200 mg twice daily. Coadministration of voriconazole at 400 mg twice daily with rifabutin 300 mg twice daily increased the C max and AUC T of rifabutin by an average of 3-times (90% CI: 2.2, 4.0) and 4 times (90% CI: 3.5, 5.4), respectively, compared to rifabutin given alone. Coadministration of voriconazole and rifabutin is contraindicated [ see Contraindications (4)].
Significant drug interactions that may require dosage adjustment, frequent monitoring of drug levels and/or frequent monitoring of drug-related adverse events/toxicity:
Efavirenz, a non-nucleoside reverse transcriptase inhibitor (CYP450 inducer; CYP3A4 inhibitor and substrate) –Standard doses of voriconazole and efavirenz (400 mg q24h or higher) must not be coadministered [see Drug Interactions (7)]. Steady state efavirenz (400 mg PO q24h) decreased the steady state C max and AUC T of voriconazole (400 mg PO q12h for 1 day, then 200 mg PO q12h for 8 days) by an average of 61% and 77%, respectively, in healthy male subjects. Voriconazole at steady state (400 mg PO q12h for 1 day, then 200 mg q12h for 8 days) increased the steady state C max and AUC T of efavirenz (400 mg PO q24h for 9 days) by an average of 38% and 44%, respectively, in healthy subjects.
The pharmacokinetics of adjusted doses of voriconazole and efavirenz were studied in healthy male subjects following administration of voriconazole (400 mg PO q12h on Days 2 to 7) with efavirenz (300 mg PO q24h on Days 1-7), relative to steady-state administration of voriconazole (400 mg for 1 day, then 200 mg PO q12h for 2 days) or efavirenz (600 mg q24h for 9 days). Coadministration of voriconazole 400 mg q12h with efavirenz 300 mg q24h, decreased voriconazole AUC T by 7% (90% CI: -23%, 13%) and increased C max by 23% (90% CI: -1%, 53%); efavirenz AUC T was increased by 17% (90% CI: 6%, 29%) and C max was equivalent.
Coadministration of standard doses of voriconazole and efavirenz (400 mg q24h or higher) is contraindicated.
Voriconazole may be coadministered with efavirenz if the voriconazole maintenance dose is increased to 400 mg q12h and the efavirenz dose is decreased to 300 mg q24h. When treatment with voriconazole is stopped, the initial dosage of efavirenz should be restored [
see
Dosage and Administration (2.4),
Contraindications (4), and
Drug Interactions (7)].
Phenytoin (CYP2C9 substrate and potent CYP450 inducer) –Repeat dose administration of phenytoin (300 mg once daily) decreased the steady state C max and AUC T of orally administered voriconazole (200 mg q12h x 14 days) by an average of 50% and 70%, respectively, in healthy subjects. Administration of a higher voriconazole dose (400 mg q12h x 7 days) with phenytoin (300 mg once daily) resulted in comparable steady state voriconazole C max and AUC T estimates as compared to when voriconazole was given at 200 mg q12h without phenytoin.
Phenytoin may be coadministered with voriconazole if the maintenance dose of voriconazole is increased from 4 mg/kg to 5 mg/kg intravenously every 12 hours or from 200 mg to 400 mg orally, every 12 hours (100 mg to 200 mg orally, every 12 hours in patients less than 40 kg) [ see Dosage and Administration (2.4), and Drug Interactions (7)].
Repeat dose administration of voriconazole (400 mg q12h x 10 days) increased the steady state C max and AUC T of phenytoin (300 mg once daily) by an average of 70% and 80%, respectively, in healthy subjects. The increase in phenytoin C max and AUC when coadministered with voriconazole may be expected to be as high as 2 times the C max and AUC estimates when phenytoin is given without voriconazole. Therefore, frequent monitoring of plasma phenytoin concentrations and phenytoin-related adverse effects is recommended when phenytoin is coadministered with voriconazole [ see Warnings and Precautions (5.1)].
Omeprazole (CYP2C19 inhibitor; CYP2C19 and CYP3A4 substrate) –Coadministration of omeprazole (40 mg once daily x 10 days) with oral voriconazole (400 mg q12h x 1 day, then 200 mg q12h x 9 days) increased the steady state C max and AUC T of voriconazole by an average of 15% (90% CI: 5%, 25%) and 40% (90% CI: 29%, 55%), respectively, in healthy subjects. No dosage adjustment of voriconazole is recommended.
Coadministration of voriconazole (400 mg q12h x 1 day, then 200 mg x 6 days) with omeprazole (40 mg once daily x 7 days) to healthy subjects significantly increased the steady state C max and AUC T of omeprazole an average of 2 times (90% CI: 1.8, 2.6) and 4 times (90% CI: 3.3, 4.4), respectively, as compared to when omeprazole is given without voriconazole. When initiating voriconazole in patients already receiving omeprazole doses of 40 mg or greater, it is recommended that the omeprazole dose be reduced by one-half [see Warnings and Precautions (5.1)].
The metabolism of other proton pump inhibitors that are CYP2C19 substrates may also be inhibited by voriconazole and may result in increased plasma concentrations of these drugs.
Oral Contraceptives (CYP3A4 substrate; CYP2C19 inhibitor) –Coadministration of oral voriconazole (400 mg q12h for 1 day, then 200 mg q12h for 3 days) and oral contraceptive (Ortho-Novum1/35® consisting of 35 mcg ethinyl estradiol and 1 mg norethindrone, q24h) to healthy female subjects at steady state increased the C max and AUC T of ethinyl estradiol by an average of 36% (90% CI: 28%, 45%) and 61% (90% CI: 50%, 72%), respectively, and that of norethindrone by 15% (90% CI: 3%, 28%) and 53% (90% CI: 44%, 63%), respectively in healthy subjects. Voriconazole C max and AUC T increased by an average of 14% (90% CI: 3%, 27%) and 46% (90% CI: 32%, 61%), respectively. Monitoring for adverse events related to oral contraceptives, in addition to those for voriconazole, is recommended during coadministration [ see Warnings and Precautions (5.1)].
No significant pharmacokinetic interaction was seen and no dosage adjustment of these drugs is recommended:
Indinavir (CYP3A4 inhibitor and substrate) –Repeat dose administration of indinavir (800 mg TID for 10 days) had no significant effect on voriconazole C max and AUC following repeat dose administration (200 mg q12h for 17 days) in healthy subjects.
Repeat dose administration of voriconazole (200 mg q12h for 7 days) did not have a significant effect on steady state C max and AUC T of indinavir following repeat dose administration (800 mg TID for 7 days) in healthy subjects.
Other Two-Way Interactions Expected to be Significant Based on In Vitro and In Vivo Findings:
Other HIV Protease Inhibitors (CYP3A4 substrates and inhibitors) – In vitro studies (human liver microsomes) suggest that voriconazole may inhibit the metabolism of HIV protease inhibitors (e.g., saquinavir, amprenavir and nelfinavir). In vitro studies (human liver microsomes) also show that the metabolism of voriconazole may be inhibited by HIV protease inhibitors (e.g., saquinavir and amprenavir). Patients should be frequently monitored for drug toxicity during the coadministration of voriconazole and HIV protease inhibitors [ see Warnings and Precautions (5.1)].
Other Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) (CYP3A4 substrates, inhibitors or CYP450 inducers) – In vitro studies (human liver microsomes) show that the metabolism of voriconazole may be inhibited by a NNRTI (e.g., delavirdine). The findings of a clinical voriconazole-efavirenz drug interaction study in healthy male subjects suggest that the metabolism of voriconazole may be induced by a NNRTI. This in vivo study also showed that voriconazole may inhibit the metabolism of a NNRTI [ see Drug Interactions (7), and Warnings and Precautions (5.9)]. Patients should be frequently monitored for drug toxicity during the coadministration of voriconazole and other NNRTIs (e.g., nevirapine and delavirdine) [ see Warnings and Precautions (5.1)]. Dose adjustments are required when voriconazole is co-administered with efavirenz [ see Drug Interactions (7), and Warnings and Precautions (5.1)].].
12.4 Microbiology
Mechanism of Action
Voriconazole is an azole antifungal agent. The primary mode of action of voriconazole is the inhibition of fungal cytochrome P-450-mediated 14 alpha-lanosterol demethylation, an essential step in fungal ergosterol biosynthesis. The accumulation of 14 alpha-methyl sterols correlates with the subsequent loss of ergosterol in the fungal cell wall and may be responsible for the antifungal activity of voriconazole.
Drug Resistance
Voriconazole drug resistance development has not been adequately studied in vitro against Candida, Aspergillus, Scedosporium and Fusarium species. The frequency of drug resistance development for the various fungi for which this drug is indicated is not known.
Fungal isolates exhibiting reduced susceptibility to fluconazole or itraconazole may also show reduced susceptibility to voriconazole, suggesting cross-resistance can occur among these azoles. The relevance of cross-resistance and clinical outcome has not been fully characterized. Clinical cases where azole cross-resistance is demonstrated may require alternative antifungal therapy.
Activity In Vitro and In Vivo
Voriconazole has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections.
Aspergillus fumigatus
Aspergillus flavus
Aspergillus niger
Aspergillus terreus
Candida albicans
Candida glabrata
(In clinical studies, the voriconazole MIC90 was 4 µg/mL)
1
Candida krusei
Candida parapsilosis
Candida tropicalis
Fusarium
spp. including
Fusarium solani
Scedosporium apiospermum
The following data are available, but their clinical significance is unknown.
Voriconazole exhibits in vitro minimal inhibitory concentrations (MICs) of 1 µg/mL or less against most (≥90%) isolates of the following microorganisms; however, the safety and effectiveness of voriconazole in treating clinical infections due to these Candida species have not been established in adequate and well-controlled clinical trials:
Candida lusitaniae
Candida guilliermondii
Susceptibility Testing Methods 1,2
Aspergillus species and other filamentous fungi
No interpretive criteria have been established for Aspergillus species and other filamentous fungi.
Candida species
The interpretive standards for voriconazole against Candida species are applicable only to tests performed using Clinical Laboratory and Standards Institute (CLSI) microbroth dilution reference method M27 for MIC read at 48 hours or disk diffusion reference method M44 for zone diameter read at 24 hours. 1,2
Broth Microdilution Techniques –Quantitative methods are used to determine antifungal minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of Candida spp. to antifungal agents. MICs should be determined using a standardized procedure at 48 hours. 1 Standardized procedures are based on a microdilution method (broth) with standardized inoculum concentrations and standardized concentrations of voriconazole powder. The MIC values should be interpreted according to the criteria provided in Table 10.
Diffusion Techniques–Qualitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of Candida spp. to an antifungal agent. One such standardized procedure requires the use of standardized inoculum concentrations. 2 This procedure uses paper disks impregnated with 1 μg of voriconazole to test the susceptibility of yeasts to voriconazole at 24 hours. Disk diffusion interpretive criteria are also provided in Table 10.
| Broth Microdilution at 48 hours
(MIC in μg/mL) | Disk Diffusion at 24 hours
(Zone diameters in mm) |
|||||
| Susceptible (S) | Intermediate (I) | Resistant (R) | Susceptible (S) | Intermediate (I) | Resistant (R) | |
| Voriconazole | ≤1.0 | 2.0 | ≥4.0 | ≥17 | 14-16 | ≤13 |
NOTE: Shown are the breakpoints (μg/mL) for voriconazole against Candida species.
A report of Susceptible (S) indicates that the antimicrobial drug is likely to inhibit growth of the microorganism if the antimicrobial drug reaches the concentration usually achievable at the site of infection. A report of intermediate (I) implies that an infection due to the isolate may be appropriately treated in body sites where the drugs are physiologically concentrated or when a high dosage of drug is used. A report of Resistant (R) indicates that the antimicrobial is not likely to inhibit growth of the pathogen if the antimicrobial drug reaches the concentrations usually achievable at the infection site; other therapy should be selected.
Quality Control
Standardized susceptibility test procedures require the use of quality control organisms to ensure the accuracy of the technical aspects of the test procedures. Standard voriconazole powder and 1 μg disks should provide the following range of values noted in Table 11.
NOTE: Quality control microorganisms are specific strains of organisms with intrinsic biological properties relating to resistance mechanisms and their genetic expression within fungi; the specific strains used for microbiological control are not clinically significant.
| ATCC is a registered trademark of the American Type Culture Collection. | ||
|
|
||
| QC Strain | Broth Microdilution (MIC in μg/mL) at 48-hour | Disk Diffusion (Zone diameter in mm) at 24-hour |
| Candida parapsilosis
ATCC 22019 | 0.03-0.25 | 28-37 |
| Candida krusei
ATCC 6258 | 0.12-1.0 | 16-25 |
| Candida albicans
ATCC 90028 | * | 31-42 |
13 NONCLINICALTOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year carcinogenicity studies were conducted in rats and mice. Rats were given oral doses of 6, 18 or 50 mg/kg voriconazole, or 0.2, 0.6, or 1.6 times the recommended maintenance dose (RMD) on a mg/m 2 basis. Hepatocellular adenomas were detected in females at 50 mg/kg and hepatocellular carcinomas were found in males at 6 and 50 mg/kg. Mice were given oral doses of 10, 30 or 100 mg/kg voriconazole, or 0.1, 0.4, or 1.4 times the RMD on a mg/m 2 basis. In mice, hepatocellular adenomas were detected in males and females and hepatocellular carcinomas were detected in males at 1.4 times the RMD of voriconazole.
Voriconazole demonstrated clastogenic activity (mostly chromosome breaks) in human lymphocyte cultures in vitro. Voriconazole was not genotoxic in the Ames assay, CHO assay, the mouse micronucleus assay or the DNA repair test (Unscheduled DNA Synthesis assay).
Voriconazole administration induced no impairment of male or female fertility in rats dosed at 50 mg/kg, or 1.6 times the RMD (recommended maintenance dose).
13.2 Teratogenic Effects
Pregnancy category D [ see Warnings and Precautions (5.4), and Use in Specific Populations (8.1)].
14 CLINICAL STUDIES
Voriconazole, administered orally or parenterally, has been evaluated as primary or salvage therapy in 520 patients aged 12 years and older with infections caused by Aspergillus spp., Fusarium spp., and Scedosporium spp.
14.1 Invasive Aspergillosis
Voriconazole was studied in patients for primary therapy of invasive aspergillosis (randomized, controlled study 307/602), for primary and salvage therapy of aspergillosis (non-comparative study 304) and for treatment of patients with invasive aspergillosis who were refractory to, or intolerant of, other antifungal therapy (non-comparative study 309/604).
Study 307/602 – Primary Therapy of Invasive Aspergillosis
The efficacy of voriconazole compared to amphotericin B in the primary treatment of acute invasive aspergillosis was demonstrated in 277 patients treated for 12 weeks in a randomized, controlled study (Study 307/602). The majority of study patients had underlying hematologic malignancies, including bone marrow transplantation. The study also included patients with solid organ transplantation, solid tumors, and AIDS. The patients were mainly treated for definite or probable invasive aspergillosis of the lungs. Other aspergillosis infections included disseminated disease, CNS infections and sinus infections. Diagnosis of definite or probable invasive aspergillosis was made according to criteria modified from those established by the National Institute of Allergy and Infectious Diseases Mycoses Study Group/European Organisation for Research and Treatment of Cancer (NIAID MSG/EORTC).
Voriconazole was administered intravenously with a loading dose of 6 mg/kg every 12 hours for the first 24 hours followed by a maintenance dose of 4 mg/kg every 12 hours for a minimum of seven days. Therapy could then be switched to the oral formulation at a dose of 200 mg q12h. Median duration of IV voriconazole therapy was 10 days (range 2-85 days). After IV voriconazole therapy, the median duration of PO voriconazole therapy was 76 days (range 2-232 days).
Patients in the comparator group received conventional amphotericin B as a slow infusion at a daily dose of 1.0-1.5 mg/kg/day. Median duration of IV amphotericin therapy was 12 days (range 1-85 days). Treatment was then continued with other licensed antifungal therapy (OLAT), including itraconazole and lipid amphotericin B formulations. Although initial therapy with conventional amphotericin B was to be continued for at least two weeks, actual duration of therapy was at the discretion of the investigator. Patients who discontinued initial randomized therapy due to toxicity or lack of efficacy were eligible to continue in the study with OLAT treatment.
A satisfactory global response at 12 weeks (complete or partial resolution of all attributable symptoms, signs, radiographic/bronchoscopic abnormalities present at baseline) was seen in 53% of voriconazole treated patients compared to 32% of amphotericin B treated patients (Table 14). A benefit of voriconazole compared to amphotericin B on patient survival at Day 84 was seen with a 71% survival rate on voriconazole compared to 58% on amphotericin B (Table 12).
Table 12 also summarizes the response (success) based on mycological confirmation and species.
|
|
|||
| Voriconazole | Ampho B * | Stratified Difference
(95% CI) † |
|
| n/N (%) | n/N (%) | ||
| Efficacy as Primary Therapy | |||
| Satisfactory Global Response ‡ | 76/144 (53) | 42/133 (32) | 21.8%
(10.5%, 33.0%) p<0.0001 |
| Survival at Day 84 § | 102/144 (71) | 77/133 (58) | 13.1%
(2.1%, 24.2%) |
| Success by Species | |||
| Success n/N (%) | |||
| Overall success | 76/144 (53) | 42/133 (32) | |
| Mycologically confirmed ¶ | 37/84 (44) | 16/67 (24) | |
| Aspergillus spp. # | |||
| A. fumigatus | 28/63 (44) | 12/47 (26) | |
| A. flavus | 3/6 | 4/9 | |
| A. terreus | 2/3 | 0/3 | |
| A. niger | 1/4 | 0/9 | |
| A. nidulans | 1/1 | 0/0 | |
Study 304 – Primary and Salvage Therapy of Aspergillosis
In this non-comparative study, an overall success rate of 52% (26/50) was seen in patients treated with voriconazole for primary therapy. Success was seen in 17/29 (59%) with Aspergillus fumigatus infections and 3/6 (50%) patients with infections due to non- fumigatus species [ A. flavus (1/1); A. nidulans (0/2); A. niger (2/2); A. terreus (0/1)]. Success in patients who received voriconazole as salvage therapy is presented in Table 13.
Study 309/604 – Treatment of Patients with Invasive Aspergillosis who were Refractory to, or Intolerant of, other Antifungal Therapy
Additional data regarding response rates in patients who were refractory to, or intolerant of, other antifungal agents are also provided in Table 15. In this non-comparative study, overall mycological eradication for culture- documented infections due to fumigatus and non- fumigatus species of Aspergillus was 36/82 (44%) and 12/30 (40%), respectively, in voriconazole treated patients. Patients had various underlying diseases and species other than A. fumigatus contributed to mixed infections in some cases.
For patients who were infected with a single pathogen and were refractory to, or intolerant of, other antifungal agents, the satisfactory response rates for voriconazole in studies 304 and 309/604 are presented in Table 13.
| Success
n/N |
|
| A. fumigatus | 43/97 (44%) |
| A. flavus | 5/12 |
| A. nidulans | 1/3 |
| A. niger | 4/5 |
| A. terreus | 3/8 |
| A. versicolor | 0/1 |
Nineteen patients had more than one species of Aspergillus isolated. Success was seen in 4/17 (24%) of these patients.
14.2 Candidemia in Non-neutropenic Patients and Other Deep Tissue Candida Infections
Voriconazole was compared to the regimen of amphotericin B followed by fluconazole in Study 608, an open label, comparative study in nonneutropenic patients with candidemia associated with clinical signs of infection. Patients were randomized in 2:1 ratio to receive either voriconazole (n=283) or the regimen of amphotericin B followed by fluconazole (n=139). Patients were treated with randomized study drug for a median of 15 days. Most of the candidemia in patients evaluated for efficacy was caused by C. albicans (46%), followed by C. tropicalis (19%), C. parapsilosis (17%), C. glabrata (15%), and C. krusei (1%).
An independent Data Review Committee (DRC), blinded to study treatment, reviewed the clinical and mycological data from this study, and generated one assessment of response for each patient. A successful response required all of the following: resolution or improvement in all clinical signs and symptoms of infection, blood cultures negative for Candida, infected deep tissue sites negative for Candida or resolution of all local signs of infection, and no systemic antifungal therapy other than study drug. The primary analysis, which counted DRC-assessed successes at the fixed time point (12 weeks after End of Therapy [EOT]), demonstrated that voriconazole was comparable to the regimen of amphotericin B followed by fluconazole (response rates of 41% and 41%, respectively) in the treatment of candidemia. Patients who did not have a 12-week assessment for any reason were considered a treatment failure.
The overall clinical and mycological success rates by Candida species in Study 150-608 are presented in Table 14.
|
|
||
| Baseline Pathogen | Clinical and Mycological Success (%) | |
| Voriconazole | Amphotericin B --> Fluconazole | |
| C. albicans | 46/107 (43%) | 30/63 (48%) |
| C. tropicalis | 17/53 (32%) | 1/16 (6%) |
| C. parapsilosis | 24/45 (53%) | 10/19 (53%) |
| C. glabrata | 12/36 (33%) | 7/21 (33%) |
| C. krusei | 1/4 | 0/1 |
In a secondary analysis, which counted DRC-assessed successes at any time point (EOT, or 2, 6, or 12 weeks after EOT), the response rates were 65% for voriconazole and 71% for the regimen of amphotericin B followed by fluconazole.
In Studies 608 and 309/604 (non-comparative study in patients with invasive fungal infections who were refractory to, or intolerant of, other antifungal agents), voriconazole was evaluated in 35 patients with deep tissue Candida infections. A favorable response was seen in 4 of 7 patients with intra-abdominal infections, 5 of 6 patients with kidney and bladder wall infections, 3 of 3 patients with deep tissue abscess or wound infection, 1 of 2 patients with pneumonia/pleural space infections, 2 of 4 patients with skin lesions, 1 of 1 patients with mixed intraabdominal and pulmonary infection, 1 of 2 patients with suppurative phlebitis, 1 of 3 patients with hepatosplenic infection, 1 of 5 patients with osteomyelitis, 0 of 1 with liver infection, and 0 of 1 with cervical lymph node infection.
14.3 Esophageal Candidiasis
The efficacy of oral voriconazole 200 mg twice daily compared to oral fluconazole 200 mg once daily in the primary treatment of esophageal candidiasis was demonstrated in Study 150-305, a double-blind, double-dummy study in immunocompromised patients with endoscopically-proven esophageal candidiasis. Patients were treated for a median of 15 days (range 1 to 49 days). Outcome was assessed by repeat endoscopy at end of treatment (EOT). A successful response was defined as a normal endoscopy at EOT or at least a 1 grade improvement over baseline endoscopic score. For patients in the Intent to Treat (ITT) population with only a baseline endoscopy, a successful response was defined as symptomatic cure or improvement at EOT compared to baseline. Voriconazole and fluconazole (200 mg once daily) showed comparable efficacy rates against esophageal candidiasis, as presented in Table 15.
|
|
|||
| Population | Voriconazole | Fluconazole | Difference %
(95% CI) * |
| PP † | 113/115 (98.2%) | 134/141 (95.0%) | 3.2 (-1.1, 7.5) |
| ITT ‡ | 175/200 (87.5%) | 171/191 (89.5%) | -2.0 (-8.3, 4.3) |
Microbiologic success rates by Candida species are presented in Table 16.
|
|
||||
| Pathogen * | Voriconazole | Fluconazole | ||
| Favorable endoscopic response † | Mycological eradication † | Favorable endoscopic response † | Mycological eradication † | |
| Success/Total (%) | Eradication/Total (%) | Success/Total (%) | Eradication/Total (%) | |
| C. albicans | 134/140 (96%) | 90/107 (84%) | 147/156 (94%) | 91/115 (79%) |
| C. glabrata | 8/8 (100%) | 4/7 (57%) | 4/4 (100%) | 1/4 (25%) |
| C. krusei | 1/1 | 1/1 | 2/2 (100%) | 0/0 |
14.4 Other Serious Fungal Pathogens
In pooled analyses of patients, voriconazole was shown to be effective against the following additional fungal pathogens:
Scedosporium apiospermum - Successful response to voriconazole therapy was seen in 15 of 24 patients (63%). Three of these patients relapsed within 4 weeks, including 1 patient with pulmonary, skin and eye infections, 1 patient with cerebral disease, and 1 patient with skin infection. Ten patients had evidence of cerebral disease and 6 of these had a successful outcome (1 relapse). In addition, a successful response was seen in 1 of 3 patients with mixed organism infections.
Fusarium spp. - Nine of 21 (43%) patients were successfully treated with voriconazole. Of these 9 patients, 3 had eye infections, 1 had an eye and blood infection, 1 had a skin infection, 1 had a blood infection alone, 2 had sinus infections, and 1 had disseminated infection (pulmonary, skin, hepatosplenic). Three of these patients (1 with disseminated disease, 1 with an eye infection and 1 with a blood infection) had Fusarium solani and were complete successes. Two of these patients relapsed, 1 with a sinus infection and profound neutropenia and 1 post surgical patient with blood and eye infections.
15 REFERENCES
- Clinical Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved Standard M27-A3. Clinical Laboratory Standards Institute, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898, USA, 2008.
- Clinical Laboratory Standards Institute (CLSI). Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts. Approved Guideline M44-A2. Clinical Laboratory Standards Institute, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898, USA, 2009.
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Tablets
VORICONAZOLE 50 mg tablets; white, round shaped, biconvex, film coated tablets with ‘APP 332’ debossed on one side and plain on other side.
Bottles of 30 (NDC: 55801-332-01)
VORICONAZOLE 200 mg tablets; white, oval shaped, biconvex, film coated tablets with ‘APP 333’ debossed on one side and plain on other side.
Bottles of 30 (NDC: 55801-333-01)
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling
Manufactured by:
Kemwell Biopharma Pvt Ltd
34th KM, Tumkur Road, T-Begur, Nelamangala
Bangalore Rural, KA, 562123, India
Distributed by:
Appco Pharma LLC
120 Belmont Drive, Somerset
New Jersey-08873
Revised: January 2016
VORICONAZOLE TABLETS
(VOR-i-KON-a-zole)
Read the Patient Information that comes with VORICONAZOLE before you start taking it and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your condition or treatment.
What is VORICONAZOLE?
VORICONAZOLE is a prescription medicine used to treat certain serious fungal infections in your blood and body. These infections are called “aspergillosis,” “esophageal candidiasis,” “ Scedosporium", ” Fusarium", and “candidemia”.
It is not known if VORICONAZOLE is safe and effective in children younger than 12 years old.
Who should not take VORICONAZOLE?
Do not take VORICONAZOLE if you:
- are allergic to voriconazole or any of the ingredients in VORICONAZOLE. See the end of this leaflet for a complete list of ingredients in VORICONAZOLE.
-
are taking any of the following medicines:
- cisapride (Propulsid ®)
- pimozide (Orap ®)
- quinidine (like Quinaglute ®)
- sirolimus (Rapamune ®)
- rifampin (Rifadin ®)
- carbamazepine (Tegretol ®)
- long-acting barbiturates like phenobarbital (Luminal ®)
- efavirenz (Sustiva ®)
- ritonavir (Norvir ®)
- rifabutin (Mycobutin ®)
- ergotamine, dihydroergotamine (ergot alkaloids)
- St. John’s Wort (herbal supplement)
Ask your healthcare provider or pharmacist if you are not sure if you are taking any of the medicines listed above. Do not start taking a new medicine without talking to your healthcare provider or pharmacist.
What should I tell my healthcare provider before taking VORICONAZOLE?
Before you take VORICONAZOLE, tell your healthcare provider if you:
- have or ever had an abnormal heart rate or rhythm. Your healthcare provider may order a test to check your heart (EKG) before starting VORICONAZOLE.
- have liver or kidney problems. Your healthcare provider may do blood tests to make sure you can take VORICONAZOLE
- have trouble digesting dairy products, lactose (milk sugar), or regular table sugar. VORICONAZOLE tablets contain lactose. VORICONAZOLE liquid contains sucrose (table sugar).
- are pregnant or plan to become pregnant. VORICONAZOLE can harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant. Women who can become pregnant should use effective birth control while taking VORICONAZOLE.
- are breast-feeding or plan to breast-feed. It is not known if VORICONAZOLE passes into breast milk. Talk to your healthcare provider about the best way to feed your baby if you take VORICONAZOLE.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements.
VORICONAZOLE may affect the way other medicines work, and other medicines may affect how VORICONAZOLE works.
Know what medicines you take. Keep a list of them to show your healthcare provider or pharmacist when you get a new medicine.
How should I take VORICONAZOLE?
VORICONAZOLE may be prescribed to you as:
- VORICONAZOLE tablets
- Take VORICONAZOLE tablets exactly as your healthcare provider tells you to.
- Take VORICONAZOLE tablets exactly as your healthcare provider tells you to.
- Take VORICONAZOLE tablets at least 1 hour before or at least 1 hour after meals.
- If you take too much VORICONAZOLE, call your healthcare provider or go to the nearest hospital emergency room.
What should I avoid while taking VORICONAZOLE?
- You should not drive at night while taking VORICONAZOLE. VORICONAZOLE can cause changes in your vision such as blurring or sensitivity to light.
- Do not drive or operate machinery, or do other dangerous activities until you know how VORICONAZOLE affects you.
- Avoid direct sunlight. VORICONAZOLE can make your skin sensitive to the sun and the light from sunlamps and tanning beds. You could get a severe sunburn. Use sunscreen and wear a hat and clothes that cover your skin if you have to be in sunlight. Talk to your healthcare provider if you get sunburn.
What are possible side effects of VORICONAZOLE?
VORICONAZOLE may cause serious side effects including:
-
liver problems. Symptoms of liver problems may include:
- itchy skin
- yellowing of your eyes
- feeling very tired
- flu-like symptoms
- nausea or vomiting
-
vision changes. Symptoms of vision changes may include:
- blurred vision
- changes in the way you see colors
- sensitivity to light (photophobia)
- serious heart problems. VORICONAZOLE may cause changes in your heart rate or rhythm, including your heart stopping (cardiac arrest).
-
allergic reactions. Symptoms of an allergic reaction may include:
- fever
- sweating
- feels like your heart is beating fast (tachycardia)
- chest tightness
- trouble breathing
- feel faint
- nausea
- itching
- skin rash
-
kidney problems. VORICONAZOLE may cause new or worse problems with kidney function, including kidney failure.
Your healthcare provider should check your kidney function while you are taking VORICONAZOLE. Your healthcare provider will decide if you can keep taking VORICONAZOLE -
serious skin reactions. Symptoms of serious skin reactions may include:
- rash or hives
- mouth sores
- blistering or peeling of your skin
- trouble swallowing or breathing
Call your healthcare provider or go to the nearest hospital emergency room right away if you have any of the symptoms listed above.
The most common side effects of VORICONAZOLE include:
- vision changes
- rash
- vomiting
- nausea
- headache
- fast heart beat (tachycardia)
- hallucinations (seeing or hearing things that are not there)
- abnormal liver function tests
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of VORICONAZOLE. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store VORICONAZOLE?
- Store VORICONAZOLE tablets and liquid at room temperature, 68° to 77° F (20° to 25°C). Do not refrigerate or freeze.
- Keep VORICONAZOLE tablets in a tightly closed container.
- Safely throw away medicine that is out of date or no longer needed.
- Keep VORICONAZOLE, as well as all other medicines, out of the reach of children.
General information about the safe and effective use of VORICONAZOLE
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use VORICONAZOLE for a condition for which it was not prescribed. Do not give VORICONAZOLE to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about VORICONAZOLE. If you would like more information, talk to your healthcare provider. You can ask your healthcare provider or pharmacist for information about VORICONAZOLE that is written for health professionals.
What are the ingredients of VORICONAZOLE?
Active ingredient:
VORICONAZOLE Inactive ingredients:
VORICONAZOLE tablets: lactose monohydrate, pregelatinized starch, colloidal silicon dioxide, croscarmellose sodium, povidone, magnesium stearate, maize starch and a coating containing hypromellose, titanium dioxide, lactose monohydrate and triacetin
Manufactured by:
Kemwell Biopharma Pvt Ltd
34th KM, Tamkur Road, T-Begur, Nelamangala,
Bangalore Rural, KA, 562123, India
Distributed by:
Appco Pharma LLC
120, Belmont Drive, Somerset
New Jersey-08873
Revised: January 2016
| VORICONAZOLE
voriconazole tablet |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| VORICONAZOLE
voriconazole tablet |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - APPCO PHARMA LLC (078510186) |
| Registrant - APPCO PHARMA LLC (078510186) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Kemwell Biopharma Private Limited | 871401927 | manufacture(55801-332, 55801-333) | |

