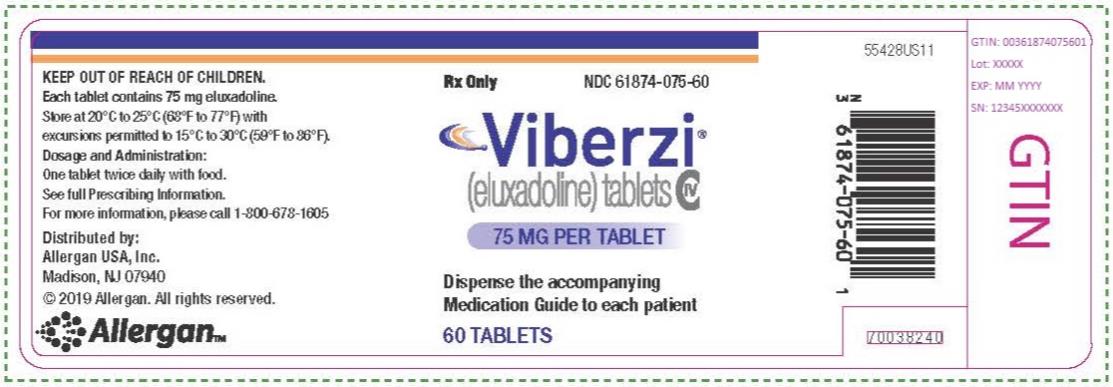
VIBERZI- eluxadoline tablet, film coated
Viberzi by
Drug Labeling and Warnings
Viberzi by is a Prescription medication manufactured, distributed, or labeled by Allergan, Inc., Patheon Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VIBERZI safely and effectively. See full prescribing information for VIBERZI.
VIBERZI (eluxadoline) tablets, for oral use, CIV
Initial U.S. Approval: 2015
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
VIBERZI is a mu-opioid receptor agonist, indicated in adults for the treatment of irritable bowel syndrome with diarrhea (IBS-D). (1)
DOSAGE AND ADMINISTRATION
- The recommended dosage in adults is 100 mg twice daily taken with food. (2)
- The recommended dosage is 75 mg twice daily taken with food in patients who:
○ are unable to tolerate the 100 mg dose. (2, 6.1)
○ are receiving concomitant OATP1B1 inhibitors. (2, 7)
○ have mild or moderate hepatic impairment. (2, 8.6)
- Discontinue VIBERZI in patients who develop severe constipation. (2)
- If a dose is missed, take the next dose at the regular time; do not take 2 doses at once. (2)
DOSAGE FORMS AND STRENGTHS
75 mg and 100 mg tablets (3)
CONTRAINDICATIONS
- patients without a gallbladder (4)
- known or suspected biliary duct obstruction, or sphincter of Oddi disease or dysfunction (4)
- alcoholism, alcohol abuse, alcohol addiction, or drink more than 3 alcoholic beverages/day (4)
- a history of pancreatitis; structural diseases of the pancreas, including known or suspected pancreatic duct obstruction (4)
- patients with a known hypersensitivity reaction to VIBERZI (4, 5.3)
- severe hepatic impairment (Child-Pugh Class C) (4, 8.6)
- a history of chronic or severe constipation or sequelae from constipation, or known or suspected mechanical gastrointestinal obstruction (4)
- alcoholism, alcohol abuse, alcohol addiction, or drink more than 3 alcoholic beverages/day (4)
WARNINGS AND PRECAUTIONS
-
Pancreatitis and Sphincter of Oddi Spasm: Monitor patients for new or worsening abdominal pain, with or without nausea and vomiting, or acute biliary pain with liver or pancreatic enzyme elevations; immediately discontinue VIBERZI and seek medical attention if symptoms develop. (5.1, 5.2)
-
Hypersensitivity Reactions, including anaphylaxis: Immediately discontinue VIBERZI and seek medical attention if symptoms develop. (4, 5.3)
- Constipation: Instruct patients to stop VIBERZI and immediately contact their healthcare provider if they develop severe constipation. Avoid use with other drugs that may cause constipation (5.4, 7)
ADVERSE REACTIONS
Most common adverse reactions (>5%) are constipation, nausea and abdominal pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Allergan at 1- 800-678-1605 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
See full prescribing information. (7)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 6/2018
- The recommended dosage in adults is 100 mg twice daily taken with food. (2)
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Pancreatitis
5.2 Sphincter of Oddi Spasm
5.3 Hypersensitivity Reactions
5.4 Constipation
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2
DOSAGE AND ADMINISTRATION
The recommended dosage of VIBERZI is 100 mg taken orally twice daily with food.
The recommended dosage of VIBERZI is 75 mg taken orally twice daily with food in patients who:
- are unable to tolerate the 100 mg dose of VIBERZI [see Adverse Reactions (6.1)].
- are receiving concomitant OATP1B1 inhibitors [see Drug Interactions (7)].
- have mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment [see Use in Specific Population (8.6), Clinical Pharmacology (12.3)].
Discontinue VIBERZI in patients who develop severe constipation [see Warnings and Precautions (5.4)].
Instruct patients if they miss a dose, take the next dose at the regular time and not to take 2 doses at the same time to make up for a missed dose.
- are unable to tolerate the 100 mg dose of VIBERZI [see Adverse Reactions (6.1)].
-
3
DOSAGE FORMS AND STRENGTHS
- 75 mg tablets: capsule-shaped tablets are coated in pale-yellow to light tan color debossed with “FX75” on one side. Each tablet contains 75 mg eluxadoline.
- 100 mg tablets: capsule-shaped tablets are coated in pink-orange to peach color debossed with “FX100” on one side. Each tablet contains 100 mg eluxadoline.
- 75 mg tablets: capsule-shaped tablets are coated in pale-yellow to light tan color debossed with “FX75” on one side. Each tablet contains 75 mg eluxadoline.
-
4
CONTRAINDICATIONS
VIBERZI is contraindicated in patients:
- Without a gallbladder. These patients are at increased risk of developing serious adverse reactions of pancreatitis and/or sphincter of Oddi spasm [see Warnings and Precautions (5.1, 5.2)]
- With known or suspected biliary duct obstruction; or sphincter of Oddi disease or dysfunction. These patients are at increased risk for sphincter of Oddi spasm [see Warnings and Precautions (5.1)].
- With alcoholism, alcohol abuse or alcohol addiction, or in patients who drink more than 3 alcoholic beverages per day. These patients are at increased risk for acute pancreatitis [see Warnings and Precautions (5.1)].
- With a history of pancreatitis; or structural diseases of the pancreas, including known or suspected pancreatic duct obstruction. These patients are at increased risk for acute pancreatitis [see Warnings and Precautions (5.1)].
- With a known hypersensitivity reaction to VIBERZI [see Warnings and Precautions (5.3)].
- With severe hepatic impairment (Child-Pugh Class C). These patients are at risk for significantly increased plasma concentrations of eluxadoline [see Use in Specific Populations (8.6)]
- With a history of chronic or severe constipation or sequelae from constipation, or known or suspected mechanical gastrointestinal obstruction. These patients may be at risk for severe complications of bowel obstruction [see Warnings and Precautions (5.4)].
- Without a gallbladder. These patients are at increased risk of developing serious adverse reactions of pancreatitis and/or sphincter of Oddi spasm [see Warnings and Precautions (5.1, 5.2)]
-
5
WARNINGS AND PRECAUTIONS
5.1 Pancreatitis
Pancreatitis, with or without sphincter of Oddi spasm [see Warnings and Precautions (5.1)], has been reported in patients taking either the 75 mg or 100 mg dosage of VIBERZI, including serious cases resulting in hospitalization, primarily in patients without a gallbladder. Fatal cases have also been reported in patients without a gallbladder. VIBERZI is contraindicated in patients without a gallbladder [see Contraindications (4)]. Most of the reported cases of serious pancreatitis occurred within a week of starting treatment with VIBERZI and some developed symptoms after one to two doses.
In patients with a gallbladder, evaluate a patient’s alcohol intake prior to starting VIBERZI. Instruct patients to avoid chronic or acute excessive alcohol use while taking VIBERZI. Monitor for new or worsening abdominal pain that may radiate to the back or shoulder, with or without nausea and vomiting. Instruct patients to immediately stop VIBERZI and seek medical attention if they experience symptoms suggestive of pancreatitis such as acute abdominal or epigastric pain radiating to the back or shoulder associated with elevations of pancreatic enzymes with or without nausea and vomiting [see Contraindications (4)].
5.2 Sphincter of Oddi Spasm
There is a risk of sphincter of Oddi spasm, resulting in pancreatitis or hepatic enzyme elevation associated with acute abdominal pain (e.g., biliary-type pain) in patients taking VIBERZI [see Adverse Reactions (6.1)]. Postmarketing serious adverse reactions of sphincter of Oddi spasm with or without pancreatitis resulting in hospitalization have been reported, primarily in patients without a gallbladder [see Warnings and Precautions (5.1)]. Most of the reported cases of serious sphincter of Oddi spasm occurred within a week of starting treatment with VIBERZI and some developed symptoms after one to two doses. VIBERZI is contraindicated in patients without a gallbladder [see Contraindications (4)].
Instruct patients to immediately stop VIBERZI and seek medical attention if they experience symptoms suggestive of sphincter of Oddi spasm such as acute worsening of abdominal pain, (e.g. acute epigastric or biliary [i.e., right upper quadrant] pain), that may radiate to the back or shoulder with or without nausea and vomiting, associated with elevations of pancreatic enzymes or liver transaminases. Do not restart VIBERZI in patients who developed biliary duct obstruction or sphincter of Oddi spasm while taking VIBERZI [see Contraindications (4)].
5.3 Hypersensitivity Reactions
In postmarketing experience, serious hypersensitivity reactions (including anaphylaxis) have been reported following VIBERZI administration. Some of these reactions occurred after the first one or two doses of VIBERZI [see Adverse Reactions (6.2)].
Instruct patients to immediately stop VIBERZI and seek medical attention if they experience symptoms suggestive of a hypersensitivity reaction [see Contraindications (4)].
5.4 Constipation
Constipation, sometimes requiring hospitalization, has been reported following VIBERZI administration. In postmarketing experience, severe cases with development of intestinal obstruction, intestinal perforation, and fecal impaction, requiring intervention, have also been reported. Instruct patients to stop VIBERZI and immediately contact their healthcare provider if they experience severe constipation. Avoid use with other drugs that may cause constipation [see Adverse Reactions (6.1), Drug Interactions (7)].
-
6
ADVERSE REACTIONS
The following adverse reactions described below and elsewhere in the labeling include:
- Pancreatitis [see Warnings and Precautions (5.1)]
- Sphincter of Oddi Spasm [see Warnings and Precautions (5.2)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- Constipation [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Over 1700 patients with IBS-D have been treated with 75 or 100 mg of VIBERZI twice daily in controlled trials. Exposures from placebo-controlled clinical trials in adult patients with IBS-D included 1391 exposed for 3 months, 1001 exposed for 6 months and 488 exposed for one year.
Demographic characteristics were comparable between the treatment groups [see Clinical Studies (14)]. Data described below represent pooled data compared to placebo across the randomized trials.
Pancreatitis
Cases of pancreatitis, not associated with sphincter of Oddi spasm, were reported in 2/807 (0.2%) of patients receiving 75 mg and 3/1032 (0.3%) of patients receiving 100 mg VIBERZI twice daily in clinical trials. Of these 5 cases, 3 were associated with excessive alcohol intake, one was associated with biliary sludge, and in one case the patient discontinued VIBERZI 2 weeks prior to the onset of symptoms. All pancreatic events resolved with lipase normalization upon discontinuation of VIBERZI, with 80% (4/5) resolving within 1 week of treatment discontinuation. The case of sphincter of Oddi spasm-induced pancreatitis resolved within 24 hours of discontinuation.
Sphincter of Oddi Spasm
In clinical trials, sphincter of Oddi spasm occurred in 0.2% (2/807) of patients receiving 75 mg and 0.8% (8/1032) of patients receiving 100 mg VIBERZI twice daily.
- Among patients receiving 75 mg, 1/807 (0.1%) patient experienced a sphincter of Oddi spasm presenting with abdominal pain but with lipase elevation less than 3 times the upper limit of normal (ULN) and 1/ 807 (0.1%) patient experienced a sphincter of Oddi spasm manifested as elevated hepatic enzymes associated with abdominal pain
- Among patients receiving 100 mg, 1/1032 (0.1%) patient experienced a sphincter of Oddi spasm manifested as pancreatitis and 7/1032 (0.7%) patients experienced sphincter of Oddi spasm manifested as elevated hepatic enzymes associated with abdominal pain
Of those patients who experienced a sphincter of Oddi spasm, 80% (8/10) reported their first onset of symptoms within the first week of treatment. The case of sphincter of Oddi spasm-induced pancreatitis occurred within minutes of taking the first dose of VIBERZI. No cases of sphincter of Oddi spasm occurred greater than 1 month after treatment onset. All events resolved upon discontinuation of VIBERZI, with symptoms typically improved by the following day.
Common Adverse Reactions
Table 1 provides the incidence of common adverse reactions reported in > 2% of IBS-D patients in either VIBERZI treatment group and at an incidence greater than in the placebo group.
Table 1: Common* Adverse Reactions in the Placebo-Controlled Studies in IBS-D Patients Adverse Reactions VIBERZI
100 mg twice daily
(N= 1032)
%VIBERZI
75 mg twice daily
(N=807)
%Placebo
(N=975)
%Constipation 8 7 2 Nausea 7 8 5 Abdominal Pain** 7 6 4 Upper Respiratory Tract Infection 5 3 4 Vomiting 4 4 1 Nasopharyngitis 3 4 3 Abdominal Distention 3 3 2 Bronchitis 3 3 2 Dizziness 3 3 2 Flatulence 3 3 2 Rash*** 3 3 2 Increased ALT 3 2 1 Fatigue 2 3 2 Viral gastroenteritis 1 3 2 * Reported in > 2% of VIBERZI-treated patients at either dose and at an incidence greater than in placebo-treated patients
**"Abdominal Pain" term includes: abdominal pain, abdominal pain lower, and abdominal pain upper
*** "Rash" term includes: dermatitis, dermatitis allergic, rash, rash erythematous, rash generalized, rash maculo-papular, rash papular, rash pruritic, urticaria, and idiopathic urticaria
Constipation was the most commonly reported adverse reaction in VIBERZI-treated patients in these trials. Approximately 50% of constipation events occurred within the first 2 weeks of treatment while the majority occurred within the first 3 months of therapy. Rates of severe constipation were less than 1% in patients receiving 75 mg and 100 mg VIBERZI. Similar rates of constipation occurred between the active and placebo arms beyond 3 months of treatment.
Adverse Reactions Leading to Discontinuation
Eight percent of patients treated with 75 mg, 8% of patients treated with 100 mg VIBERZI and 4% of patients treated with placebo discontinued prematurely due to adverse reactions. In the VIBERZI treatment groups, the most common reasons for discontinuation due to adverse reactions were constipation (1% for 75 mg and 2% for 100 mg) and abdominal pain (1% for both 75 mg and 100 mg). In comparison, less than 1% of patients in the placebo group withdrew due to constipation or abdominal pain.
Less Common Adverse Reactions
Adverse reactions that were reported in ≤ 2% of VIBERZI-treated patients are listed below by body system.
Gastrointestinal: gastroesophageal reflux disease
General Disorders and administration site conditions: feeling drunk
Investigations: increased AST
Nervous system: sedation, somnolence
Psychiatric disorders: euphoric mood
Respiratory: asthma, bronchospasm, respiratory failure, wheezing6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of VIBERZI. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hypersensitivity: anaphylaxis, angioedema (e.g. swollen face and throat), dyspnea, throat tightness, and chest pain/tightness [see Warnings and Precautions (5.3)].
- Pancreatitis [see Warnings and Precautions (5.1)]
-
7
DRUG INTERACTIONS
Tables 2 and 3 include drugs which demonstrated a clinically important drug interaction with VIBERZI or which potentially may result in clinically relevant interactions.
Table 2: Established and Other Potentially Clinically Relevant Interactions Affecting VIBERZI OATP1B1 Inhibitors Clinical Impact: Increased exposure to eluxadoline when coadministered with cyclosporine [see Clinical Pharmacology (12.3)] Intervention: Administer VIBERZI at a dose of 75 mg twice daily [see Dosage and Administration (2)] and monitor patients for impaired mental or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery and for other eluxadoline-related adverse reactions [see Adverse Reactions (6.1)]. Examples: cyclosporine, gemfibrozil, antiretrovirals (atazanavir, lopinavir, ritonavir, saquinavir, tipranavir), rifampin, eltrombopag Drugs that Cause Constipation Clinical Impact: Increased risk for constipation related adverse reactions and potential for constipation related serious adverse reactions Intervention: Avoid use with other drugs that may cause constipation (see below); loperamide may be used occasionally for acute management of severe diarrhea but avoid chronic use. Discontinue loperamide immediately if constipation occurs. Examples: alosetron, anticholinergics, opioids Table 3: Established and Other Potentially Clinically Relevant Interactions Affecting Drugs Co-Administered with VIBERZI OATP1B1 and BCRP Substrate Clinical Impact: VIBERZI may increase the exposure of co-administered OATP1B1 and BCRP substrates.
Increased exposure to rosuvastatin when co-administered with VIBERZI with a potential for increased risk of myopathy/rhabdomyolysis [see Clinical Pharmacology (12.3)]Intervention: Use the lowest effective dose of rosuvastatin (see prescribing information of rosuvastatin for additional information on recommended dosing). -
8
USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no studies with VIBERZI in pregnant women that inform any drug-associated risks. The background risk of major birth defects and miscarriage for the indicated population is unknown. However, the background risk in the U.S. general population of major birth defects is 2 to 4% and of miscarriage is 15 to 20% of clinically recognized pregnancies. In animal reproduction studies, oral and subcutaneous administration of eluxadoline to rats and rabbits during organogenesis at doses approximately 51 and 115 times the human exposure after a single oral dose of 100 mg, respectively, demonstrated no teratogenic effects. In a pre- and postnatal development study in rats, no adverse effects were observed in offspring with oral administration of eluxadoline at doses approximately 10 times the human exposure [see Data].
Data
Animal Data
Eluxadoline administered as combined oral (1000 mg/kg/day) and subcutaneous (5 mg/kg/day) doses during the period of organogenesis to rats and rabbits (exposures about 51 and 115 times, respectively, the human AUC of 24 ng.h/mL after a single oral dose of 100 mg) did not cause any adverse effects on embryofetal development. A pre- and postnatal development study in rats showed no evidence of any adverse effect on pre- and postnatal development at oral doses of eluxadoline up to 1000 mg/kg/day (with exposures about 10 times the human AUC of 24 ng.h/mL after a single oral dose of 100 mg). In the same study, eluxadoline was detected in the milk of lactating rats administered oral doses of 100, 300 and 1000 mg/kg/day (with exposures about 1.8, 3 and 10 times, respectively, the human AUC of 24 ng.h/mL after a single oral dose of 100 mg). Milk samples were collected from six lactating females per group on lactation day 12. Mean concentrations of eluxadoline in the milk of lactating rats on lactation day 12 were 2.78, 5.49 and 44.02 ng/mL at 100, 300 and 1000 mg/kg/day, respectively.
8.2 Lactation
Risk Summary
No data are available regarding the presence of eluxadoline in human milk, the effects of eluxadoline on the breastfed infant, or the effects of eluxadoline on milk production. However, eluxadoline is present in rat milk [see Use in Specific Populations (8.1)].
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for VIBERZI and any potential adverse effects on the breastfed infant from VIBERZI or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Juvenile Toxicology Data
Eluxadoline was orally administered to juvenile rats at 500, 750, and 1500 mg/kg/day (about 16, 54 and 30 times, respectively, the human AUC of 24 ng.h/mL after a single oral dose of 100 mg) for 4 weeks. There were no adverse physiologic effects related to eluxadoline. Based on these results, the NOAEL for male and female juvenile rats was 1500 mg/kg/day (about 30 times the human AUC of 24 ng.h/mL after a single oral dose of 100 mg).
8.5 Geriatric Use
Of 1795 IBS-D patients in clinical trials of VIBERZI who received 75 mg or 100 mg twice daily, 139 (7.7%) were at least 65 years of age, while 15 (0.8%) were at least 75 years old. No overall differences in effectiveness were observed between these patients and younger patients. There were no overall differences in the types of adverse reactions observed between elderly and younger patients; however, a higher proportion of elderly patients than younger patients experienced adverse reactions (66% vs 59%), serious adverse reactions (9% vs 4%), and gastrointestinal adverse reactions (39% vs 28%).
8.6 Hepatic Impairment
Plasma concentrations of eluxadoline increase in patients with hepatic impairment [see Clinical Pharmacology (12.3)].
VIBERZI is contraindicated in patients with severe hepatic impairment (Child-Pugh Class C) as plasma concentrations of eluxadoline increase significantly (16-fold) and there is no information to support the safety of VIBERZI in these patients.
In patients with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment, plasma concentrations of eluxadoline increase to a lesser extent (6- and 4-fold, respectively). Administer VIBERZI at a reduced dose of 75 mg twice daily to these patients [see Dosage and Administration (2)]. Monitor patients with any degree of hepatic impairment for impaired mental or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery and for other eluxadoline-related adverse reactions [see Adverse Reactions (6.1)].
-
9
DRUG ABUSE AND DEPENDENCE
9.2 Abuse
In a drug discrimination study in monkeys, intravenous administration of eluxadoline hydrochloride produced full generalization to the morphine cue. In a self-administration study in monkeys, eluxadoline hydrochloride was self-administered to a degree that was less than that of heroin but greater than that of saline.
Adverse reactions of euphoria and feeling drunk were reported in clinical trials of IBS-D evaluating 75 mg and 100 mg doses of VIBERZI. The rate of euphoria was 0% for 75 mg and 0.2% (2/1032) for 100 mg and the rate of feeling drunk was 0.1% (1/807) for 75 mg and 0.1% (1/1032) for 100 mg.
In contrast, in two human abuse potential studies conducted in recreational opioid-experienced individuals, supratherapeutic oral doses of VIBERZI (300 mg and/or 1000 mg) and intranasal doses of VIBERZI (100 mg and/or 200 mg) produced the adverse reaction of euphoria (at a rate ranging from 14% to 28%) that was greater than that of placebo (0% to 5%) but less than that of oxycodone (44% to 76%). In the two human abuse potential studies, supratherapeutic oral and intranasal doses of VIBERZI produced small but significant increases on positive subjective measures such as Drug Liking and High compared to placebo. Supratherapeutic oral and intranasal doses of VIBERZI also produced small but significant increases on negative subjective measures such as Drug Disliking and Dysphoria compared to placebo. In the same studies, oxycodone (30 mg and 60 mg oral, and 15 and 30 mg intranasal) produced significantly greater responses on positive and negative subjective measures than those produced by eluxadoline and placebo.
9.3 Dependence
In studies with monkeys and rats in which eluxadoline and eluxadoline hydrochloride were chronically administered, discontinuation of the drug did not lead to behavioral signs of withdrawal, a measure of physical dependence. However, the ability of eluxadoline hydrochloride in monkeys to induce self-administration suggests that the drug is sufficiently rewarding to produce reinforcement. In two human abuse potential studies with VIBERZI conducted in recreational opioid-experienced individuals, euphoria was reported at a rate of 14% to 28%. These data suggest that eluxadoline may produce psychological dependence.
-
10
OVERDOSAGE
No reports of overdosage with VIBERZI have been reported.
In the event of acute overdose, the stomach should be emptied and adequate hydration maintained. The patient should be carefully observed and given standard supportive treatment as required. Given eluxadoline’s action at opioid receptors, administration of a narcotic mu-opioid antagonist, such as naloxone, should be considered. Considering the short half-life of naloxone, repeated administration may be necessary. In the event of naloxone administration, subjects should be monitored closely for the return of overdose symptoms, which may indicate need for repeated naloxone injection.
-
11
DESCRIPTION
The active ingredient in VIBERZI is eluxadoline, a mu-opioid receptor agonist.
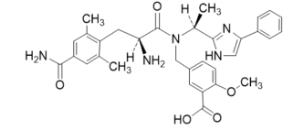
The full chemical name is 5-[[[(2S)-2-amino-3-[4-(aminocarbonyl)-2,6-dimethylphenyl]-1-oxopropyl][(1S)-1-(4-phenyl-1H-imidazol-2-yl)ethyl]amino]methyl]-2-methoxybenzoic acid.
Eluxadoline has a molecular weight of 569.65 and a molecular formula of C32H35N5O5. The chemical structure of eluxadoline is:
VIBERZI is available as 75 mg and 100 mg tablets for oral administration. In addition to the active ingredient, eluxadoline, each tablet contains the following inactive ingredients: silicified microcrystalline cellulose, colloidal silica, crospovidone, mannitol, magnesium stearate, and Opadry II (partially hydrolyzed polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, iron oxide yellow, and iron oxide red).
-
12
CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Eluxadoline is a mu-opioid receptor agonist; eluxadoline is also a delta opioid receptor antagonist and a kappa opioid receptor agonist. The binding affinities (Ki) of eluxadoline for the human mu and delta opioid receptors are 1.8 nM and 430 nM, respectively. The binding affinity (Ki) of eluxadoline for the human kappa opioid receptor has not been determined; however, the Ki for guinea pig cerebellum kappa opioid receptor is 55 nM. In animals, eluxadoline interacts with opioid receptors in the gut.
12.2 Pharmacodynamics
Cardiac Electrophysiology
At a dose 10 times the maximum recommended dose (100 mg), VIBERZI does not prolong the QT interval to any clinically relevant extent.
12.3 Pharmacokinetics
Following oral administration of 100 mg VIBERZI in healthy subjects, the Cmax of eluxadoline was approximately 2 to 4 ng/mL and AUC was 12 to 22 ng.h/mL. Eluxadoline has approximately linear pharmacokinetics with no accumulation upon repeated twice daily dosing. The variability of eluxadoline pharmacokinetic parameters ranges from 51% to 98%.
Absorption
Absolute bioavailability of eluxadoline has not been determined.
Effect of Food
The median Tmax value was 1.5 hours (range: 1 to 8 hours) under fed conditions and 2 hours (range: 0.5 to 6 hours) under fasting conditions [see Dosage and Administration (2)].
The administration of VIBERZI with a high fat meal that contained approximately 800 to 1000 total calories, with 50% of calories being derived from fat content decreased the Cmax of eluxadoline by 50% and AUC by 60%.
Distribution
Plasma protein binding of eluxadoline was 81%.
Elimination
The mean plasma elimination half-life of eluxadoline ranged from 3.7 hours to 6 hours.
Metabolism
Cytochrome P450 (CYP) and UGT pathways have minimal involvement in the metabolism of eluxadoline. It is unlikely that metabolism of eluxadoline by these enzymes has a clinically meaningful impact on systemic exposure.
Excretion
Following a single oral dose of 300 mg [14C] eluxadoline in healthy male subjects, 82.2% of the total radioactivity was recovered in feces within 336 hours and less than 1% was recovered in urine within 192 hours.
Specific Populations
Patients with Hepatic Impairment
Following a single oral 100–mg dose in subjects with varying degrees of liver impairment and healthy subjects, mean eluxadoline plasma exposure was 6-fold, 4-fold, and 16-fold higher in mild, moderate, and severe hepatically impaired subjects (Child Pugh Class A, B, C), respectively, compared to the subjects with normal liver function [see Dosage and Administration (2), Contraindications (4), Use in Specific Populations (8.6)].
Drug Interaction Studies
In Vitro Assessment of Drug Interactions
In vitro studies indicate that eluxadoline is neither an inducer of CYP1A2, CYP2C9, CYP2C19, and CYP3A4, nor an inhibitor of CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2C19, and CYP2D6 at clinically relevant systemic concentrations. Although CYP2E1 was slightly inhibited by eluxadoline (IC50 of approximately 20 micromolar [11 mcg/mL]), clinically meaningful interactions are unlikely. Despite mechanism-based inhibition of CYP3A4 in vitro, eluxadoline did not have a clinically meaningful drug-drug interaction with the CYP3A4 substrate midazolam.
In vitro studies suggest that eluxadoline is a substrate for OAT3, OATP1B1, BSEP and MRP2, but not for OCT1, OCT2, OAT1, OATP1B3, P-gp and BCRP. Based on the in vitro studies, clinically meaningful interaction via inhibition of OCT1, OCT2, OAT1, OAT3, OATP1B3, BSEP and MRP2 by eluxadoline is unlikely.
In Vivo Assessment of Drug Interactions
The following drug interactions were studied in healthy subjects:
Oral Contraceptives
Coadministration of multiple doses of 100 mg VIBERZI with multiple dose administration of an oral contraceptive (norethindrone 0.5 mg/ethinyl estradiol 0.035 mg) does not change the exposure of either drug.
Cyclosporine
Coadministration of a single dose of 100 mg VIBERZI with a single dose of 600 mg cyclosporine resulted in 4.4-fold and 6.2-fold increase in AUC and Cmax of eluxadoline, respectively, compared to administration of VIBERZI alone [see Drug Interactions (7)].
Probenecid
Coadministration of a single dose of 100 mg VIBERZI with a single dose of 500 mg probenecid resulted in a 35% and 31% increase in eluxadoline AUC and Cmax, respectively, compared to administration of VIBERZI alone. This change in eluxadoline exposures is not expected to be clinically meaningful.
Rosuvastatin
Coadministration of multiple doses of 100 mg VIBERZI twice daily with a single dose 20 mg rosuvastatin resulted in an increase in the AUC (40%) and Cmax (18%) of rosuvastatin compared to administration of rosuvastatin alone. Similar results were observed with the active, major metabolite, n-desmethyl rosuvastatin [see Drug Interactions (7)].
Midazolam
Coadministration of multiple doses of 100 mg VIBERZI twice daily with single dose administration of 4 mg midazolam did not affect midazolam pharmacokinetics in humans, suggesting that eluxadoline will not affect the exposure of concomitantly administered CYP3A4 substrates.
-
13
NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Two-year oral carcinogenicity studies have been conducted with eluxadoline in CD-1 mice at doses up to 1500 mg/kg/day (about 14 times the human AUC of 24 ng.h/mL after a single oral dose of 100 mg) and in Sprague Dawley rats at oral doses up to 1500 mg/kg/day (about 36 times the human AUC of 24 ng.h/mL after a single oral dose of 100 mg). Oral administration of eluxadoline for 104 weeks did not produce tumors in mice and rats.
Mutagenesis
Eluxadoline was negative in the Ames test, chromosome aberration test in human lymphocytes, in the mouse lymphoma cell (L5178Y/TK+/-) forward mutation test and in the in vivo rat bone marrow micronucleus test.
Impairment of Fertility
Eluxadoline at oral doses up to 1000 mg/kg/day (about 10 times the human AUC of 24 ng.h/mL after a single oral dose of 100 mg) was found to have no adverse effect on fertility and reproductive performance of male and female rats.
-
14
CLINICAL STUDIES
The efficacy and safety of VIBERZI in IBS-D patients was established in two randomized, multi-center, multi-national, double-blind, placebo-controlled trials (Studies 1 and 2). A total of 1281 patients in Study 1 and 1145 patients in Study 2 received treatment with VIBERZI 75 mg, VIBERZI 100 mg or placebo twice daily [overall, patients had a mean age of 45 years (range 18 to 80 years with 10% at least 65 years of age or older), 66% female, 86% white, 11% black, and 27% Hispanic].
All patients met Rome III criteria for IBS-D (loose [mushy] or watery stools ≥25% and hard or lumpy stools <25% of bowel movements) and were required to meet both of the following criteria:
- an average of worst abdominal pain scores in the past 24 hours of >3.0 on a 0 to 10 scale over the week prior to randomization.
- an average daily stool consistency score (Bristol Stool Scale or BSS) of ≥5.5 and at least 5 days with a BSS score ≥5 on a 1 to 7 scale over the week prior to randomization.
Pertinent exclusion criteria included: prior pancreatitis, alcohol abuse, cholecystitis prior 6 months, sphincter of Oddi dysfunction, inflammatory bowel disease, intestinal obstruction, gastrointestinal infection or diverticulitis within prior 3 months, lipase greater than 2 xULN, ALT or AST greater than 3 xULN.
Study 1 and Study 2 included identical 26-week double-blind, placebo-controlled treatment periods. Study 1 continued double-blinded for an additional 26 weeks for long-term safety (total of 52 weeks of treatment), followed by a 2-week follow-up. Study 2 included a 4-week single-blinded, placebo-withdrawal period upon completion of the 26-week treatment period. During the double-blind treatment phase and the single-blinded placebo withdrawal phase, patients were allowed to take loperamide rescue medication for the acute treatment of uncontrolled diarrhea, but were not allowed to take any other antidiarrheal, antispasmodic agent or rifaximin for their diarrhea. Additionally, patients were allowed to take aspirin-containing medications or nonsteroidal anti-inflammatory drugs, but no narcotic or opioid containing agents.
Efficacy of VIBERZI was assessed in both trials using an overall composite responder primary endpoint. The primary endpoint was defined by the simultaneous improvement in the daily worst abdominal pain score by ≥30% as compared to the baseline weekly average AND a reduction in the BSS to <5 on at least 50% of the days within a 12-week time interval. Improvement in daily worst abdominal pain in the absence of a concurrent bowel movement was also considered a response day. Results for endpoints were based on electronic daily diary entries by patients.
The proportion of composite responders over 12 weeks is shown in Table 4. In both trials, the proportion of patients who were composite responders to VIBERZI was statistically significantly higher than placebo for both doses. The proportion of patients who were composite responders to VIBERZI was similar for male and female patients in both trials.
Table 4: Efficacy Results in Randomized Clinical Trials Study 1 Study 2 VIBERZI 100mg
twice daily
n=426VIBERZI 75mg
twice daily
n=427PBO
n=427VIBERZI
100mg
twice daily
n=382VIBERZI
75mg
twice daily
n=381PBO n=382 Composite1 Response
over 12 weeksResponder rates 25% 24% 17% 30% 29% 16% Treatment difference 8%2 7%4 13%3 13%3 95% CI (%) (2.6, 13.5) (1.4, 12.2) (7.5, 19.2) (6.8, 18.5) Composite Response
over 26 weeksResponder rates 29% 23% 19% 33% 30% 20% Treatment difference 10% 4% 13% 10% 95% CI (%) (4.7, 16.1) (-1.0, 9.9) (6.4, 18.8) (4.2, 16.4) Abdominal Pain Response Improved ≥30%
over 12 weeksResponder rates 43% 42% 40% 51% 48% 45% Treatment difference 4% 3% 6% 3% 95% CI (%) (-3.0, 10.2) (-3.8, 9.4) (-1.3, 12.8) (-4.3, 9.8) BSS <5 Response
over 12 weeksResponder rates 34% 30% 22% 36% 37% 21% Treatment difference 12% 8% 15% 16% 95% CI (%) (6.3, 18.2) (2.1, 13.8) (8.4, 21.0) (9.7, 22.4) 1Composite= Simultaneous improvement of Worst Abdominal Pain (WAP) by ≥30% and Bristol Stool Score (BSS) < 5 on the same day for ≥ 50% of days over the interval
2 P<0.01
3 P<0.001
4 P<0.05
Additionally, the proportion of patients who were composite responders to VIBERZI at each 4-week interval was numerically higher than placebo for both doses as early as month 1 through month 6 demonstrating that efficacy is maintained throughout the course of treatment.
During the 4 week single-blind withdrawal period in Study 2, no evidence of worsening of diarrhea or abdominal pain compared to baseline was demonstrated at either dose.
- an average of worst abdominal pain scores in the past 24 hours of >3.0 on a 0 to 10 scale over the week prior to randomization.
-
16
HOW SUPPLIED/STORAGE AND HANDLING
VIBERZI is available as:
- 75 mg tablets: capsule-shaped tablets, coated in pale-yellow to light tan color, debossed with “FX75” on one side.
Bottle of 60: NDC: 61874-075-60
- 100 mg tablets: capsule-shaped tablets, coated in pink-orange to peach color, debossed with “FX100” on one side.
Bottle of 60: NDC: 61874-100-60
Store VIBERZI tablets at 20°C to 25°C (68°F to 77°F) with excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
- 75 mg tablets: capsule-shaped tablets, coated in pale-yellow to light tan color, debossed with “FX75” on one side.
-
17
PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Instruct patients to:
- stop VIBERZI immediately and seek medical attention if unusual or severe abdominal pain that may radiate to the back or shoulder with or without nausea and vomiting, develops [see Warnings and Precautions (5.1, 5.2)].
- avoid chronic or acute excessive alcohol use while taking VIBERZI [see Warnings and Precautions (5.1)].
- stop VIBERZI immediately and seek medical attention if symptoms of a hypersensitivity reaction develop [see Warnings and Precautions (5.3)].
- stop VIBERZI and immediately call their health care provider if they experience severe constipation [see Dosage and Administration (2), Warnings and Precautions (5.4)].
- take one tablet twice daily with food.
- if they miss a dose, take the next dose at the regular time. Do not take 2 doses at the same time to make up for a missed dose.
- call their healthcare provider if they are unable to tolerate VIBERZI
- not take alosetron with VIBERZI or not take loperamide on a chronic basis with VIBERZI due to the potential for constipation. Loperamide may occasionally be used with VIBERZI for acute management of severe diarrhea, but must be discontinued if constipation develops. Also, instruct patients to avoid taking VIBERZI with other medications that may cause constipation (for example opioids, anticholinergics, etc.) [see Drug Interactions (7)].
Distributed by:
Allergan USA, Inc.
Madison, NJ 07940© 2018 Allergan. All rights reserved.
V1.0USPI0075
- stop VIBERZI immediately and seek medical attention if unusual or severe abdominal pain that may radiate to the back or shoulder with or without nausea and vomiting, develops [see Warnings and Precautions (5.1, 5.2)].
-
MEDICATION GUIDE
MEDICATION GUIDE
VIBERZI (vye BER zee), CIV
(eluxadoline) tabletsWhat is the most important information I should know about VIBERZI?
VIBERZI can cause serious side effects, including:
-
Inflammation of the pancreas (pancreatitis). Pancreatitis has happened most often in people who do not have a gallbladder and can lead to hospitalization. Pancreatitis has led to death in some people who do not have a gallbladder. Pancreatitis usually happens within the first week of treatment with VIBERZI but can happen after 1 to 2 doses of VIBERZI. Your risk of getting pancreatitis is increased if you drink more than 3 alcoholic drinks a day. Limit your use of alcoholic drinks while you are taking VIBERZI.
- Sphincter of Oddi spasm. The sphincter of Oddi is a muscular valve that controls the flow of digestive juices (bile and pancreatic juice) to the first part of your small intestine. A sphincter of Oddi spasm can cause an increase in your liver and pancreas enzymes and inflammation of the pancreas (pancreatitis) that can cause sudden stomach-area (abdomen) pain. Sphincter of Oddi spasm has happened most often in people who do not have a gallbladder and can lead to hospitalization. This spasm usually happens within the first week of treatment with VIBERZI but can happen after 1 or 2 doses of VIBERZI.
- Serious allergic reactions. Serious allergic reactions have happened in some people after taking 1 or 2 doses of VIBERZI. Stop taking VIBERZI right away and get emergency medical care if you have signs or symptoms of an allergic reaction, including:
○ swelling of your face, lips, mouth or tongue ○ itching ○ shortness of breath or other breathing problems ○ rash ○ hives - Constipation. Constipation, including severe constipation that can lead to hospitalization has happened after taking VIBERZI. Stop taking VIBERZI and call your doctor right away if you develop severe constipation while taking VIBERZI.
What is VIBERZI?
VIBERZI is a prescription medicine used to treat adults who have irritable bowel syndrome with diarrhea (IBS-D).
- VIBERZI is a controlled substance (CIV) because it contains eluxadoline and may be abused or lead to drug dependence. Keep your VIBERZI in a safe place, to protect it from theft. Never give your VIBERZI to anyone else, because it may harm them. Selling or giving away this medicine is against the law.
People 65 years old and older have had an increased number of side effects, including serious side effects and stomach problems, while taking VIBERZI than people younger than 65 years old have had.Do not take VIBERZI if you:
- do not have a gallbladder.
- have or may have had a blockage in your gallbladder or a sphincter of Oddi problem.
- have or had problems with alcohol abuse, alcohol addiction, or drink more than 3 alcoholic drinks a day.
- have had inflammation of your pancreas (pancreatitis) or other pancreas problems, including if you have had or may have had a blockage in your pancreas.
- have had an allergic reaction to VIBERZI.
- have severe liver problems.
- have had long-lasting (chronic) or severe constipation, or problems caused by constipation.
- have or may have had a bowel blockage (intestinal obstruction).
Before taking VIBERZI, tell your doctor about all of your medical conditions, including if you:
- See “What is the most important information I should know about VIBERZI?”
- have liver problems.
- are pregnant or plan to become pregnant. It is not known if VIBERZI will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if VIBERZI passes into your breast milk or could harm your baby.
If you are taking VIBERZI you should not take:
- medicines that cause constipation including:
○ Lotronex® (alosetron)
○ anticholinergic medicines
○ opioid pain medicines
- Avoid taking loperamide, a medicine used to treat diarrhea, for a long time (chronic use). You may take loperamide occasionally to treat severe diarrhea. Stop taking loperamide right away if you become constipated.
How should I take VIBERZI? - Take VIBERZI exactly as your doctor tells you to take it.
- Take 1 tablet of VIBERZI 2 times each day with food.
- If you miss a dose, take your next dose at your regular time. Do not take 2 doses at the same time to make up for a missed dose.
- Do not change your dose or stop taking VIBERZI unless your doctor tells you to.
- If you take too much VIBERZI, call your doctor or go to the nearest hospital emergency room right away.
What should I avoid while taking VIBERZI? - Limit your use of alcoholic drinks while you are taking VIBERZI.
- If you have liver problems, do not drive, operate machinery, or do other dangerous activities until you know how VIBERZI affects you.
What are the possible side effects of VIBERZI?
See “What is the most important information I should know about VIBERZI?”
The most common side effects of VIBERZI include: constipation, nausea, and abdominal pain. Stop taking VIBERZI right away and immediately call your doctor if you have constipation that is severe.
These are not all the possible side effects of VIBERZI. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store VIBERZI?
Store VIBERZI at room temperature between 68°F to 77°F (20°C to 25°C).
Keep VIBERZI and all medicines out of the reach of children.General Information about the safe and effective use of VIBERZI.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use VIBERZI for a condition for which it was not prescribed. Do not give VIBERZI to other people, even if they have the same symptoms that you have. It may harm them. You can ask your doctor or pharmacist for information about VIBERZI that is written for health professionals.What are the ingredients in VIBERZI?
Active ingredient: eluxadoline
Inactive ingredients: silicified microcrystalline cellulose, colloidal silica, crospovidone, mannitol, magnesium stearate, and Opadry II (partially hydrolyzed polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, iron oxide yellow, and iron oxide red).
Distributed by: Allergan USA, Inc. Madison, NJ 07940
© 2018 Allergan. All rights reserved.
For more information, go to www.VIBERZI.com or call 800-678-1605.This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: April 2018
-
Inflammation of the pancreas (pancreatitis). Pancreatitis has happened most often in people who do not have a gallbladder and can lead to hospitalization. Pancreatitis has led to death in some people who do not have a gallbladder. Pancreatitis usually happens within the first week of treatment with VIBERZI but can happen after 1 to 2 doses of VIBERZI. Your risk of getting pancreatitis is increased if you drink more than 3 alcoholic drinks a day. Limit your use of alcoholic drinks while you are taking VIBERZI.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VIBERZI
eluxadoline tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 61874-075 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ELUXADOLINE (UNII: 45TPJ4MBQ1) (ELUXADOLINE - UNII:45TPJ4MBQ1) ELUXADOLINE 75 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE (UNII: 2S7830E561) MANNITOL (UNII: 3OWL53L36A) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color YELLOW (yellow) Score no score Shape CAPSULE (CAPSULE) Size 17mm Flavor Imprint Code FX75 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61874-075-60 60 in 1 BOTTLE 10/01/2015 1 NDC: 61874-075-08 1 in 1 CARTON 1 8 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206940 10/01/2015 VIBERZI
eluxadoline tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 61874-100 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ELUXADOLINE (UNII: 45TPJ4MBQ1) (ELUXADOLINE - UNII:45TPJ4MBQ1) ELUXADOLINE 100 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE (UNII: 2S7830E561) MANNITOL (UNII: 3OWL53L36A) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color PINK (pink) Score no score Shape CAPSULE (CAPSULE) Size 19mm Flavor Imprint Code FX100 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61874-100-60 60 in 1 BOTTLE 10/01/2015 1 NDC: 61874-100-08 1 in 1 CARTON 1 8 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206940 10/01/2015 Labeler - Allergan, Inc. (144796497) Establishment Name Address ID/FEI Business Operations Patheon Pharmaceuticals Inc. 005286822 LABEL(61874-075, 61874-100) , MANUFACTURE(61874-075, 61874-100) , PACK(61874-075, 61874-100)
Trademark Results [Viberzi]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VIBERZI 86803941 4982924 Live/Registered |
ALLERGAN HOLDINGS UNLIMITED COMPANY 2015-10-29 |
 VIBERZI 86481014 4928665 Live/Registered |
ALLERGAN HOLDINGS UNLIMITED COMPANY 2014-12-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.