PSORIZIDE FORTE- nickel sulfate, potassium bromide, and fumaric acid tablet
Psorizide Forte by
Drug Labeling and Warnings
Psorizide Forte by is a Homeopathic medication manufactured, distributed, or labeled by PLYMOUTH HEALTHCARE PRODUCTS LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- CAUTION
-
DESCRIPTION
PSORIZIDE® Forte is a biochemical homeopathic medication indicated for the treatment of contact dermatitis due to nickel (metal/jewelry allergy), dyshidrotic hand/foot eczema, and mild to severe psoriasis.1-3 The active ingredients in each PSORIZIDE® Forte tablet consist of the following: Fumaric Acid (Fumaricum Acidum) 1X, Potassium Bromide (Kali Bromatum) 1X, and Nickel Sulphate (Niccolum Sulphuricum) 1X. These drug ingredients are listed in the Homoeopathic Pharmacopoeia of the United States (HPUS).4
Inactive ingredients: Lactose and Magnesium Stearate.
Pharmacological Class: Homeopathic drug.
Dosage form: Oral 600 mg scored tablet. May be swallowed whole, chewed or dissolved in the mouth and swallowed.
-
CLINICAL PHARMACOLOGY
The active ingredients in PSORIZIDE® Forte are simple biochemical compounds. The exact mechanism of action is unknown; however, it is believed PSORIZIDE® Forte addresses a primary genetic biochemical defect.5
FUMARIC ACID is a naturally occurring four carbon organic acid important in the Krebs cycle. This biochemical pathway is of central importance to energy production. Each tablet contains approximately 30 mg fumaric acid (calculated). Fumaric Acid has many uses, including use as a food additive (GRAS) and as a chelating agent.13 The use of fumaric acid and its derivatives (esters) as a treatment for psoriasis is increasing worldwide.14-20 Very little is known about its clinical pharmacology; however, dose dependant inhibitory effects on keratinocyte proliferation have been demonstrated. 16,21
POTASSIUM BROMIDE dissolves and dissociates in the digestive tract into its ionic constituents. Each tablet contains approximately 15 mg bromide (calculated). Ionic bromide is rapidly and completely absorbed from the intestine and distributed almost exclusively into the extracellular fluids.11,12 Bromide is eliminated by the kidneys and the elimination half-life is 11-12 days. "Once a day" dosing will lead to a steady state concentration in about seven weeks.11
NICKEL SULPHATE dissolves and dissociates in the digestive tract into its ionic constituents. Each tablet contains approximately 1.0 mg of ionic nickel (calculated). According to studies, 15% to 50% of ionic nickel is absorbed on a fasted stomach.6 Food markedly decreases the rate and extent of nickel absorption.7,8 Clinical studies show that serum concentrations of nickel are variable among patients after administering the same dose.9 Peak serum nickel concentration is reached about two hours after oral administration. "Once a day" dosing leads to steady state serum concentrations in approximately one week. Nickel is in its highly stable divalent cation state and is therefore not expected to be metabolized to any significant degree in the body. Absorbed nickel is primarily excreted in the urine and elimination half-life is about 21 hours.7,9 Renal clearance is rapid and efficient, and nickel does not accumulate in the body.10
- CLINICAL STUDIES
-
INDICATIONS
PSORIZIDE® Forte is indicated for the treatment of contact dermatitis due to nickel (metal/jewelry allergy,) dyshidrotic hand/foot eczema, and mild to severe psoriasis. It has been found to work well with a variety of combination therapies. Eczema, seborrhea and a variety of chronic pruritic inflammatory dermatoses generally respond well also.
-
CONTRAINDICATIONS
Although there are no known contraindications, patients who are allergic to any PSORIZIDE® Forte ingredient should consult a physician prior to taking the medication. (Refer to Section on Hypersensitivity)
-
WARNING
Do not use if imprinted seal under bottle cap is missing or broken. Do not use if pregnant or nursing. If allergic to nickel or metal objects such as jewelry, see PRECAUTIONS for hypersensitivity information. Lactose intolerant patients may have gastrointestinal difficulty. This has very rarely been reported at the doses used.
-
PRECAUTIONS
Carefully adjust dosage to weight when treating young children. Use cautiously in setting of kidney disease. (see Dosage and Administration) If skin rash appears or if nervous symptoms persist, recur frequently, or are unusual, discontinue use.
Hypersensitivity
Caution should be used when administering to patients with a history of contact sensitivity to nickel (common metal exposure). Nickel allergy may be confirmed by a positive nickel patch test. Most patients with positive nickel allergy history or a positive nickel patch test do not have any untoward reaction to administration of PSORIZIDE® Forte. However, if there is a history of nickel sensitivity, begin with a very low dose and slowly increase over a period of six weeks as tolerated. Progressive G.I. absorption allows desensitization to occur.8
Nickel desensitization schedule: Week Amount of Time to Take Medication Prior to Breakfast Week 1 1 tablet With Breakfast Week 2 1 tablet 15 min Prior Week 3 1 tablet 30 min Prior Week 4 1 tablet 45 min Prior Week 5 1 tablet 1 hour Prior Week 6 and thereon 2 tablets 1 hour Prior If new pruritic rashes occur or persist, discontinue PSORIZIDE® Forte and treat appropriately. Do not use if there is a history of extra-cutaneous hypersensitivity to nickel or any ingredient in PSORIZIDE® Forte.
Information for patients
Patients using PSORIZIDE® Forte should receive the following information and instructions:
- This medication is to be used only as directed by a physician.
- It is important to take orally at the beginning of the day on an empty stomach (or any convenient time after having taken nothing but water for at least 7 hours) and to eat or drink nothing but water for one hour afterwards to avoid interference with absorption.
Carcinogenesis, mutagenesis, and impairment of fertility
No studies have been done on the carcinogenesis, mutagenesis, or impairment of fertility of PSORIZIDE® Forte. No carcinogenesis or mutagenesis has been reported in multiple animal studies for oral administration of fumaric acid and soluble nickel and bromide salts (active ingredients) even at very high doses.24-27
Effects of fumaric acid
It is not listed as a carcinogen by ACGIH, IARC, NIOSH, NTP, or OSHA.26 It is a GRAS substance (generally recognized as safe) and is commonly used in food processing.
Effects of soluble potassium bromide
KBr is not listed as a carcinogen by the NTP, IARC, and OSHA.28
Effects of soluble nickel sulphate
Studies on experimental animals have never indicated that nickel, at any dose, is a carcinogen when introduced to the body orally. Furthermore, Nickel sulphate and other highly water soluble nickel salts, have never been known to induce carcinogenesis via any route of introduction including: oral, inhalation, cutaneous, IM, or IP.10-12,27 No adverse effects were noted on fertility or reproduction in a 3-generational study of albino Wistar rats fed up to 1000 ppm Ni per day, which is equivalent to 50 mg/kg body weight per day Ni.27
-
ADVERSE REACTIONS
PSORIZIDE® Forte contains low doses of active ingredients. Therefore there are minimal known side effects.
(see PRECAUTIONS for hypersensitivity information)
-
OVERDOSAGE
Fumaric acid toxicity
The oral rat LD50 is 9300mg/kg.26 This is 3800 times the maximum dose recommended for PSORIZIDE® Forte.
Potassium bromide toxicity
Indications of toxicity due to oral overdosage of bromide may include nausea and vomiting, apathy, disturbed coordination, loss of memory, drowsiness, loss of emotional control, agitation, hallucination, tremors, depressed reflexes, stupor, and coma. Acute toxic reactions in humans have been reported at doses as low as 1000mg.31 This level is 67 times the dose received in one tablet of PSORIZIDE® Forte.
Nickel sulphate toxicity
The oral rat LD50 for nickel sulphate hexahydrate is 275mg/kg.29 Symptoms of toxicity due to oral overdosage of nickel sulphate may include nausea, vomiting, abdominal discomfort, diarrhea, giddiness, lassitude, headaches, cough, and shortness of breath.30 The lowest observed transitory toxic effects from human ingestion of soluble nickel salts is approximately 8 mg nickel/kg body weight.30 This is 138 times the maximum dose recommended for PSORIZIDE® Forte (see below).
-
DOSAGE AND ADMINISTRATION
Absorption of nickel sulphate is variable among individuals. For maximum absorption, tablets should be taken orally at the beginning of the day (or any convenient time after having taken nothing but water for at least 7 hours). Take nothing but water for one hour after taking medication to aid absorption.
Weight Starting Dose Max Daily Dose 40-80 lbs ½ tablet 1 ½ tablet 80-120 lbs 1 tablet 3 tablets 120-160 lbs 1 ½ tablets 4 ½ tablets 160-200 lbs 2 tablets 6 tablets 200-240 lbs 2 ½ tablets 7 ½ tablets Over 240 lbs 3 tablets 9 tablets In the setting of renal impairment
Dosage should be adjusted and serum nickel and bromide levels should be followed. Steady state trough level should be drawn prior to ingesting the day's dose after one week of dosing or at appropriate intervals. Target trough serum nickel level is 30-60 mcg/L. (Caution: post dose peak levels are unreliable.)
Maintenance phase
In order to maintain symptomatic relief, medication may be continued at the same or reduced initial phase dose level. Treatment duration depends on the individual. For allergic nickel dermatitis, continue 2 tablets 1 hour prior to breakfast for weeks 7 – 16. (Refer to Hypersensitivity section above). Some patients may require continued or intermittent repeated treatment to maintain nickel desensitization.
- INACTIVE INGREDIENTS
-
HOW SUPPLIED
Scored tablets, off white in color with green speckles, with
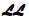 imprinted on one side and a score on the other, in child-resistant and tamperresistant bottles of 90. NDC: 61480-255-05
imprinted on one side and a score on the other, in child-resistant and tamperresistant bottles of 90. NDC: 61480-255-05
-
REFERENCES
- Reckeweg, Hans-Heinrich, Materia Medica, 1983, first English edition.
- Boericke, William, Materia Medica with Reperatory, 1927, ninth edition.
- Clarke, John Henry, A Dictionary of Practical Materia Medica, 1921, reprint edition 1996.
- The Homeopathic Pharmacopoeia of the United States (HPUS), December 2000 edition, Falls Church, Virginia.
- Kiehl R, Ionescu G, A Defective Purine Nucleotide Synthesis Pathway in Psoriatic Patients, Acta Derm Venereol (stockh), 1992, 72:253-255.
- Sunderman FW Jr. Biological monitoring of nickel in humans. Scand J. Work Environ Health 1993; 19 suppl 1:34-38.
- Sunderman FW Jr., Hopfer SM, Sweeney KR, Marcus AH, Most BM, Creason J. Ncikel absorption and kinetics in human volunteers. P.S.E.B.M. 1989; 191:5-11.
- Solomons NW, Viteri F, Shuler TR, Nielsen FH, Bioavailability of nickel in man: Effects of foods and chemically-defined dietary constituents on the absorption of inorganic nickel. J Nutr 1982; 112:39-50.
- Christensen OB, Lagesson V. Nickel concentration of blood and urine after oral administration. Annals of Clinical and Laboratory Science 1981;2(2);119-125.
- Nielsen FH. Is nickel nutritionally important? Nutrition Today 1993; 28 (1):14-19.
- Vaiseman N. Koren G. Pencharz P. Pharmacokinetics of oral and intravenous bromide in normal volunteers. Clinical Toxicology 1986;24(5):403-413.
- Van Leeuwen FXR, Sangster B. The toxicology of bromide ion. CRC Critical Reviews in Toxicology 1987; 18(3): 189-213.
- Wade A, Weller PJ Handbook of Pharmaceutical Excipients 2nd edition, American Pharmaceutical Association, 1994.
- Parfitts K, editor, Martindale: The complete Drug Reference, 1999, 32nd edition, Pharmaceutical Press, London.
- Nieboer C, et al, Fumaric Acid Therapy in Psoriasis, Dermatologica, 1990; 181:33-37
- Mrowietz U, et al, Treatment of severe psoriasis with fumaric acid esters: scientific background and guidelines for therapeutic use, Br J Dermatol 1999;141:424-429.
- Mrowietz U, et al, Treatment of psoriasis with fumaric acid esters, Br J Dermatol 1998; 138:456-460.
- Kolbach DN, Nieboer C, Fumaric Acid Therapy in Psoriasis, J Am Acad Dermatol 1992; 27: 769-771.
- Altmeyer P, et al, Antipsoriatic Effect of Fumaric Acid Derivatives, J Am Acad Dermatol 1994; 30:997-981.
- Nugteren – Huying WM, etal, Fumaric Acid Therapy for Psoriasis, J Am Acad Dermatol 1990; 22: 311-312.
- Thio, HB, et al, Fumaric Acid Derivatives… Inhibit the proliferation of Human Keratinocytes, Br J Dermatol 1994; 131: 856-866.
- Smith SA. Oral supplementation of nickel and bromide in psoriasis vulgaris using nickel sulfate and sodium bromide. 1995 (unpublished report).
- Smith SA, et al, Improvement of Psoriasis Vulgaris with Oral Nickel Dibromide. Archives of Dermatology 1997; 133:661-663.
- US EPA, Environmental Criteria and Assessment Office, Office of Health and Environmental Assessment. Drinking water quantification of toxicological effects for nickel. ECAO-CIN-443. US FDA, Center for Food Safety and Applied Nutrition. Guidance document for nickel in shellfish. 1993.
- IARC Monograph on the Evaluation of Carcinogenic Risk of Chemicals to Man. IRC Publication #11, Lyon, France 1976.
- MSDS Fumaric Acid. Acros Organics, Fair Lawn, N.J., Revised 8/2000.
- Ambrose AM, Larson PS, Borsellaca FJ, and Hennigar GR, JR. Long term toxicologic assessment of nickel in rats and dogs. Journal of Food and Science and Technology 1976;13:181-187.
- MSDS Sheet No. 247 Potassium Bromide, Schenectady, NY: Genium Publishing Corp 1991.
- MSDS Sheet No. 37 Nickel Sulfate, Schenectady, NY: Genium Publishing Corp 1993.
- Sunderman FW Jr., Dingle B, Hopfer SM, and Swift T. Acute nickel toxicity in electroplating workers who accidentally ingested a solution of nickel sulfate and nickel chloride. American Journal of Industrial Medicine 1988; 14:257-266.
- Martindale: The Extra Pharmacopoeia, 27th ed. Wade A, editor. The Pharmaceutical Press: London, 1977, pp 273-274.
- Smith SA, Baker AE, etal. Effective Treatment of Seborrheic Dermatitis Using a Low Dose, Oral Homeopathic Medication… Alt Med Rev 2002; 7, pp59-67, 1991.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 90 Tablet Label
NDC: 61480-255-05
Homeopathic MedicationPSORIZIDE® Forte
Indicated for treatment of
PSORIASIS90 Tablets

-
INGREDIENTS AND APPEARANCE
PSORIZIDE FORTE
nickel sulfate, potassium bromide, and fumaric acid tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 61480-255 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Nickel Sulfate (UNII: 4FLT4T3WUN) (NICKEL CATION - UNII:OIS2CXW7AM) Nickel Sulfate 1 [hp_X] Potassium Bromide (UNII: OSD78555ZM) (BROMIDE ION - UNII:952902IX06) Potassium Bromide 1 [hp_X] Fumaric Acid (UNII: 88XHZ13131) (Fumaric Acid - UNII:88XHZ13131) Fumaric Acid 1 [hp_X] Product Characteristics Color WHITE (Off-White with Green Speckles) Score 2 pieces Shape ROUND Size 11mm Flavor Imprint Code LL Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61480-255-05 90 in 1 BOTTLE; Type 0: Not a Combination Product 11/15/2001 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved homeopathic 11/15/2001 Labeler - PLYMOUTH HEALTHCARE PRODUCTS LLC (079330314) Establishment Name Address ID/FEI Business Operations MEDINATURA, INC. 102783016 Manufacture(61480-255)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.