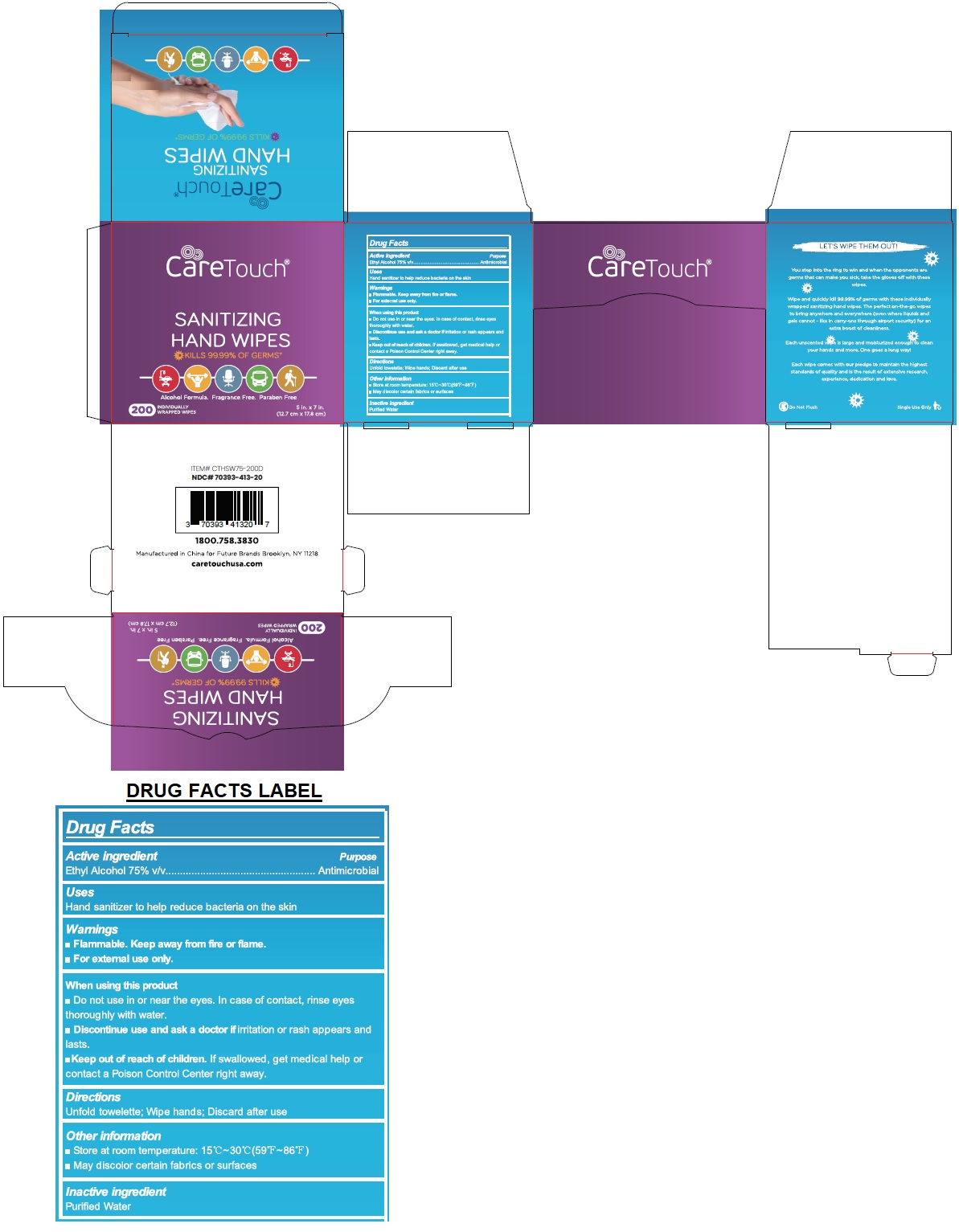
CareTouch® SANITIZING HAND WIPES
CareTouch SANITIZING HAND WIPES by
Drug Labeling and Warnings
CareTouch SANITIZING HAND WIPES by is a Otc medication manufactured, distributed, or labeled by Future Diagnostics Llc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CARETOUCH SANITIZING HAND WIPES- alcohol cloth
Future Diagnostics Llc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
CareTouch® SANITIZING HAND WIPES
Warnings
Flammable. Keep away from fire or flame.
For external use only.
When using this product
Do not use in or near the eyes. In case of contact, rinse eyes thoroughly with water.
Discontinue use and ask a doctor if irritation or rash appears and lasts.
Other information
Store at room temperature: 15°C ~ 30°C (59°F ~86°F)
May discolor certain fabrics or surfaces
KILLS 99.99% OF GERMS*
Alcohol Formula. Fragrance Free. Paraben Free
Manufactured in China for Future Brands Brooklyn, NY 11218
caretouchusa.com
LET'S WIPE THEM OUT!
You step into the ring to win and when the opponents are germs that can make you sick, take the gloves off with these wipes.
Wipe and quickly kill 99.99% of germs with these individually wrapped sanitizing hand wipes. The perfect on-the-go wipes to bring anywhere and everywhere (even where liquids and gels cannot - like in carry-ons through airport security) for an extra boost of cleanliness.
Each unscented wipe is large and moisturized enough to clean your hands and more. One goes a long way!
Each wipe comes with our pledge to maintain the highest standards of quality and is the result of extensive research, experience, dedication and love.
Do Not Flush
Single Use Only
| CARETOUCH SANITIZING HAND WIPES
alcohol cloth |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Future Diagnostics Llc (080113296) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.