SALICOR- triethanolamine salicylate patch
Salicor by
Drug Labeling and Warnings
Salicor by is a Prescription medication manufactured, distributed, or labeled by Clinic Pharma. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Salicor™ is an external analgesic product containing 10% triethanolamine salicylate as the active ingredient. Triethanolamine salicylate is an organic compound formed between triethanolamine and salicylic acid, where triethanolamine neutralizes the acidity of salicylic acid. This external analgesic is designed for temporary relief of minor pain associated with arthritis, simple backache, muscle strains, sprains, and bruises. Unlike other external analgesics, triethanolamine salicylate has no distinct odor, which improves patient acceptability.
- HOW SUPPLIED
-
DOSAGE AND ADMINISTRATION
For adults 18 years and older:
Clean and dry the affected area
Locate the tear notch on the edge of the pouch. Tear open at the notch or carefully cut open the pouch with scissors, taking care not to cut the system inside
Remove the transparent release liner before applying Salicor™ to the skin
Apply one Salicor™ to the affected area of pain and leave it in place for 8 to 12 hours
Apply only one Salicor™ at a time
If pain persists, the used Salicor™ may be replaced with a new one for up to 8 to 12 more hours
Always remove and properly dispose of the used Salicor™ before applying a new one
Salicor™ may be cut into smaller sizes with scissors prior to removing the release liner
Safely discard the used Salicor™ (whole or cut pieces) where children and pets cannot access it
Wash hands with soap and water after applying or removing Salicor™Visual Guide provided below:
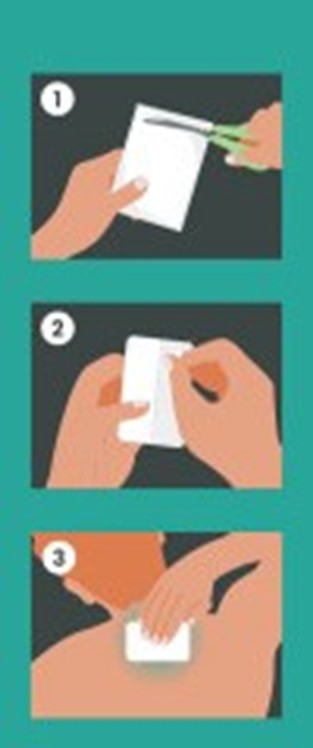
These highlights do not include all the information needed to use Salicor™ safely and effectively. See full prescribing information for Salicor™
- STORAGE AND HANDLING
-
Pharmacodynamics
Triethanolamine salicylate is an external analgesic that functions by inhibiting cyclooxygenase (COX) enzymes, which are involved in the production of pro-inflammatory factors such as prostaglandins and thromboxanes. These enzymes play a crucial role in generating pain and inflammation in conditions like arthritis, muscle strains, and sprains. While salicylates, such as aspirin, are known to inhibit COX enzymes, the specific mechanism of triethanolamine salicylate in topical applications may differ slightly. It is generally believed to act similarly to topical NSAIDs, reducing inflammation and pain locally. However, the evidence regarding its selectivity towards COX-2 enzymes is less clear.
-
Drugs That May Cause Methemoglobinemia When Used with Salicor™
Patients who are administered local anesthetics are at increased risk of developing methemoglobinemia when concurrently exposed to the following drugs, which could include other local anesthetics:
Examples of Drugs Associated with Methemoglobinemia
Class: Nitrates/Nitrites: Local anesthetics: Antineoplastic agents: Antibiotics: Antimalarials: Anticonvulsants: Other drugs: Examples: nitric oxide, nitroglycerin, nitroprusside, nitrous oxide articaine, benzocaine, bupivacaine, lidocaine, mepivacaine, prilocaine, procaine, ropivacaine, tetracaine cyclophosphamide, flutamide, hydroxyurea, ifosfamide, rasburicase dapsone, nitrofurantoin, para-aminosalicylic acid, sulfonamides chloroquine, primaquine phenobarbital, phenytoin, sodium valproate acetaminophen, metoclopramide, quinine, sulfasalazine carcinogenesis, mutagenesis -
Pharmacokinetics
Absorption
Following external administration of Salicor™ to healthy volunteers, no detectable levels of salicylic acid were found in the serum, indicating low systemic absorption. This minimal absorption is advantageous as it reduces the risk of systemic side effects commonly associated with oral salicylates. Studies have shown that urinary recovery of total salicylate during the first 24 hours after external application was only 6.9 mg, which represents approximately 1.4% of the total dose applied.
Distribution
Triethanolamine salicylate is transported and distributed within cells and tissues through various mechanisms. Studies in canines and humans have shown that transdermal absorption of salicylate from triethanolamine salicylate preparations applied to skin is consistent and reproducible, with measurable tissue salicylate levels at the application site. The distribution appears to be concentration-dependent, with tissue salicylate levels directly proportional to the concentrations of the active ingredient in the formulation up to the 10% preparation.
Metabolism
While specific metabolism data for externally applied Salicor™ is limited due to its low systemic absorption, the small amount that does enter the systemic circulation is likely metabolized similarly to other salicylates. The primary metabolic pathways include:
1. Conjugation with glycine to form salicyluric acid (approximately 75%).
2. Conjugation with glucuronic acid to produce salicyl acyl and phenolic glucuronides (about 15%).
3. Oxidation to gentisic acid and other hydroxylated derivatives (minor pathway).
Free, unmodified salicylate accounts for 10–30% of excreted metabolites, depending on dosage and individual factors like urinary pH. Metabolism primarily occurs in the liver, with some possible local metabolism in skin tissues.Excretion
Triethanolamine salicylate is primarily excreted through the urine after metabolism. Following external application of a 10% formulation, approximately 1.4% of the total dose is recovered in the urine as salicylate within the first 24 hours, indicating low systemic absorption. -
INDICATION AND USAGE
Salicor™ is indicated for the temporary relief of minor aches and pains of muscles and joints associated with: arthritis, simple backache, muscle strains and sprains, bruises, bursitis, and dysmenorrhea.
Salicor™ provides a localized analgesic effect directly at the site of pain, offering convenience and sustained relief compared to cream formulations. -
PRECAUTIONS
General
If irritation or a burning sensation occurs during application, wash the product off your skin and do not reapply until the irritation subsides.
When Salicor™ is used concomitantly with other products containing local anesthetic agents, the amount absorbed from all formulations must be considered.Stop use and ask a doctor if
Condition worsens
Symptoms persist for more than 7 days, or symptoms return within a few days after discontinuing use
Redness is present
Irritation developsHepatic Disease
Patients with severe hepatic disease are at greater risk of developing toxic blood concentrations of triethanolamine salicylate because of their inability to metabolize triethanolamine salicylate normally.Allergic Reactions
Although rare, allergic reactions to oral or external triethanolamine salicylate may occur. Seek emergency medical help if you experience hives, difficulty breathing, or swelling of the face, lips, tongue, or throat, as these may indicate a serious allergic reaction.Non-intact Skin
Although not tested, application to broken or inflamed skin may result in higher blood concentrations of triethanolamine salicylate from increased absorption. Salicor™ is only recommended for use on intact skin.External Heat Sources
Placement of external heat sources, such as heating pads or electric blankets, over Salicor™ is not recommended, as this has not been evaluated and may increase plasma triethanolamine salicylate levels.
Eye Exposure
Although not studied, contact of Salicor™ with the eyes should be avoided based on the findings of severe eye irritation with the use of similar animal products. If eye contact occurs, immediately wash out the eye with water or saline and protect the eye until sensation returns.Information for Patients Methemoglobinemia
Inform patients that the use of local anesthetics may cause methemoglobinemia, a serious condition that must be treated promptly. Advise patients or caregivers to stop use and seek immediate medical attention if they or someone in their care experiences the following signs or symptoms: pale, gray, or blue colored skin (cyanosis); headache; rapid heart rate; shortness of breath; lightheadedness; or fatigue. -
OVERDOSAGE
Salicor™ is intended for external use only. While systemic toxicity is unlikely with proper use, accidental ingestion or excessive application may lead to salicylate toxicity. Symptoms of overdose may include: nausea and vomiting, tinnitus (ringing in the ears), dizziness and confusion, rapid breathing (hyperventilation), sweating, headache, and fever.
In severe cases, overdose may lead to more serious symptoms such as: seizures, hallucinations, respiratory distress, kidney failure, and metabolic acidosis.
If overdose is suspected, discontinue use immediately and seek medical attention. Treatment is supportive and symptomatic. Healthcare professionals may need to monitor fluid and electrolyte balance, correct acid-base disturbances, and manage any complications. -
Pregnancy
Before taking NSAIDs, tell your healthcare provider about all of your medical conditions, including if you are pregnant or plan to become pregnant. Taking NSAIDs at about 20 weeks of pregnancy or later may harm your unborn baby. If you need to take NSAIDs for more than 2 days when you are between 20 and 30 weeks of pregnancy, your healthcare provider may need to monitor the amount of fluid in your womb around your baby. You should not take NSAIDs after about 30 weeks of pregnancy.
Pregnancy Category B.
Salicor™ has not been studied in pregnancy. - Labor and Delivery
- Lactation
- Pediatric Use
- Patient Package Insert
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SALICOR
triethanolamine salicylate patchProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 83881-501 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TEA-SALICYLATE (UNII: H8O4040BHD) (SALICYLIC ACID - UNII:O414PZ4LPZ) TEA-SALICYLATE 40.2 mg Inactive Ingredients Ingredient Name Strength 2-ETHYLHEXYL ACRYLATE-METHYL ACRYLATE-GLYCIDYL METHACRYLATE-ACRYLIC ACID COPOLYMER (FOR DURO-TAK 387-2353) (UNII: 737PT7E2CY) HEPTANE (UNII: 456148SDMJ) KOLLIDON SR (UNII: S34RY76LK6) ISOPROPYL ALCOHOL (UNII: ND2M416302) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 83881-501-15 15 in 1 PACKAGE; Type 0: Not a Combination Product 10/20/2027 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/22/2025 Labeler - Clinic Pharma (119158469)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.



