NAPHAZOLINE HYDROCHLORIDE AND PHENIRAMINE MALEATE solution/ drops
Naphazoline Hydrochloride and Pheniramine Maleate by
Drug Labeling and Warnings
Naphazoline Hydrochloride and Pheniramine Maleate by is a Otc medication manufactured, distributed, or labeled by Akorn, Inc., Akorn, Inc, Akorn, Inc. . Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Uses
-
Warnings:
For external use only
Ask a doctor before use if you have
- heart disease
- high blood pressure
- narrow angle glaucoma
- trouble urinating
When using this product
- pupils may become enlarged temporarily causing light sensitivity
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
- remove contact lenses before using
- do not use if this solution changes color or become cloudy
- overuse may cause more eye redness
- some users may experience a brief tingling sensation
- Directions
- Other information
- Inactive ingredients
- Questions?
-
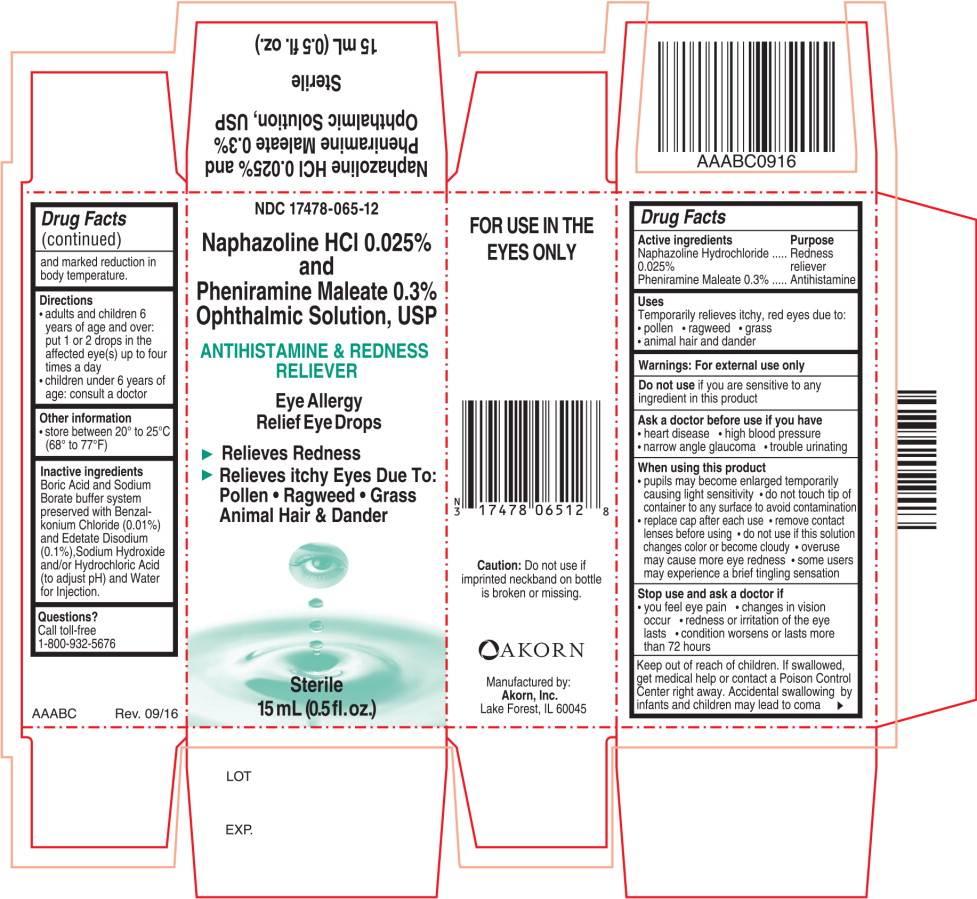
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
NDC: 17478-065-12
Naphazoline HCl 0.025%
and
Pheniramine Maleate 0.3%
Ophthalmic Solution, USP
ANTIHISTAMINE & REDNESS
RELIEVER
Eye Allergy
Relief Eye Drops
Sterile
15 mL (0.5 fl. oz.)
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Carton Label:
NDC: 17478-065-12
Naphazoline HCl 0.025%
and
Pheniramine Maleate 0.3%
Ophthalmic Solution, USP
ANTIHISTAMINE & REDNESS
RELIEVER
Eye Allergy
Relief Eye Drops
► Relieves Redness
► Relieves itchy Eyes Due To:
Pollen ● Ragweed ● Grass
Animal Hair & Dander
Sterile
15 mL (0.5 fl. oz.)
-
INGREDIENTS AND APPEARANCE
NAPHAZOLINE HYDROCHLORIDE AND PHENIRAMINE MALEATE
naphazoline hydrochloride and pheniramine maleate solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 17478-065 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Naphazoline Hydrochloride (UNII: MZ1131787D) (Naphazoline - UNII:H231GF11BV) Naphazoline Hydrochloride 0.25 mg in 1 mL Pheniramine Maleate (UNII: NYW905655B) (Pheniramine - UNII:134FM9ZZ6M) Pheniramine Maleate 3 mg in 1 mL Inactive Ingredients Ingredient Name Strength Boric Acid (UNII: R57ZHV85D4) Sodium Borate (UNII: 91MBZ8H3QO) Benzalkonium Chloride (UNII: F5UM2KM3W7) Edetate Disodium (UNII: 7FLD91C86K) Sodium Hydroxide (UNII: 55X04QC32I) Hydrochloric Acid (UNII: QTT17582CB) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17478-065-12 1 in 1 CARTON 01/24/2013 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202795 01/24/2013 Labeler - Akorn, Inc. (062649876) Establishment Name Address ID/FEI Business Operations Akorn, Inc 063434679 PACK(17478-065) , LABEL(17478-065) Establishment Name Address ID/FEI Business Operations Akorn, Inc. 155135783 MANUFACTURE(17478-065) , ANALYSIS(17478-065) , STERILIZE(17478-065)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.