NOVOLIN R- human insulin injection, solution
Novolin by
Drug Labeling and Warnings
Novolin by is a Otc medication manufactured, distributed, or labeled by TYA Pharmaceuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Novolin R safely and effectively. See full prescribing information for Novolin R. Initial U.S. Approval: 1991
Novolin R (Regular, Human Insulin [rDNA origin] USP) solution for subcutaneous or intravenous use ®
RECENT MAJOR CHANGES
Warnings and Precautions ( ) 3/2013 5.9
INDICATIONS AND USAGE
Novolin R is a short-acting recombinant human insulin indicated to improve glycemic control in adults and children with diabetes mellitus ( ). 1
DOSAGE AND ADMINISTRATION
- The dosage and timing of Novolin R must be individualized ( ). 2.1
- Subcutaneous injection: Administer approximately 30 minutes prior to the start of a meal ( ). 2.2
- Intravenous use: Use at concentrations from 0.05 to 1.0 Unit/mL in infusion systems using polypropylene infusion bags. Novolin R is stable in 0.9% sodium chloride, 5% dextrose, or 10% dextrose with 40 mmol/L potassium chloride ( ). 2.3
- Use in pumps: Not recommended due to risk of precipitation ( ). 2.4
DOSAGE FORMS AND STRENGTHS
Novolin R, Regular, Human Insulin Injection (rDNA origin) USP, 100 units/mL (U-100), is supplied in 10 mL vials ( ). 3
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Hypoglycemia: Most common adverse reaction of insulin therapy and may be life-threatening. Closely monitor blood glucose. Changes in insulin or dosage should be made cautiously and only under medical supervision ( ). 5.2
- Hypokalemia: Particularly when insulin is given intravenously or in settings of poor glycemic control. Use caution in patients predisposed to hypokalemia ( ). 5.3
- Renal or hepatic impairment: As with other insulins, the dose requirements for Novolin R may be reduced ( ). 5.5
- Allergic reactions: Severe, life-threatening, generalized allergy, including anaphylaxis, may occur ( ). 5.6
- Mixing: Do not mix Novolin R with any insulin for intravenous use. Do not mix with insulins other than NPH insulin for subcutaneous use ( ). 5.7
- Fluid retention and heart failure can occur with concomitant use of thiazolidinediones (TZDs), which are PPAR-gamma agonists, and insulin, including Novolin R ( ). 5.9
ADVERSE REACTIONS
Adverse reactions observed with Novolin R include hypoglycemia, allergic reactions, injection site reactions, lipodystrophy, weight gain and edema ( ). 6
To report SUSPECTED ADVERSE REACTIONS, contactNovo Nordisk Inc. at 1-800-727-6500or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2014
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Treatment of Diabetes Mellitus
2 DOSAGE AND ADMINISTRATION
2.1 Dosing
2.2 Subcutaneous Injection
2.3 Intravenous Use
2.4 Use in Insulin Pumps
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Administration
5.2 Hypoglycemia
5.3 Hypokalemia
5.4 Hyperglycemia, Diabetic Ketoacidosis, and Hyperosmolar Hyperglycemic Non-Ketotic Syndrome
5.5 Renal or Hepatic Impairment
5.6 Hypersensitivity and Allergic Reactions
5.7 Mixing of Insulins
5.8 Antibody Production
5.9 Fluid retention and heart failure with concomitant use of PPAR-gamma agonists
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Type 1 diabetes mellitus (adults)
14.2 Type 2 diabetes mellitus (adults)
14.3 Type 1 diabetes mellitus (children and adolescents)
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Recommended Storage
17 PATIENT COUNSELING INFORMATION
17.1 Instructions for All Patients
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosing
The dosage and timing of Novolin R must be individualized. Blood glucose monitoring is essential for all patients receiving insulin therapy.
Total daily insulin requirements vary and are usually between 0.5 and 1.0 units/kg/day. Insulin requirements may be altered during stress, major illness, or with changes in exercise, meal patterns, or coadministered medications.
2.2 Subcutaneous Injection
Novolin R should generally be injected approximately 30 minutes prior to the start of a meal.
Novolin R given by subcutaneous injection should generally be used in regimens that include an intermediate or long-acting insulin . [see How Supplied/Storage and Handling ( , )] 16.116.2
Novolin R should be administered by subcutaneous injection in the abdominal region, buttocks, thigh, or the upper arm. Subcutaneous injection into the abdominal wall is generally associated with faster absorption than other injection sites. Injection sites should be rotated within the same region to reduce the risk of lipodystrophy. Injection into a lifted skin fold minimizes the risk of intramuscular injection.
2.3 Intravenous Use
Novolin R can be administered intravenously under medical supervision for glycemic control, with close monitoring of blood glucose and potassium concentrations to avoid hypoglycemia and hypokalemia . [see Warnings and Precautions ( , ), How Supplied/Storage and Handling ( , )] 5.25.316.116.2
Intravenous administration of insulin is commonly used in the treatment of diabetic ketoacidosis, peri-operative management of diabetes, and maintenance of glycemic control during labor in pregnant diabetic women. For intravenous use, Novolin R should be used at concentrations from 0.05 units/mL to 1.0 unit/mL in infusion systems using polypropylene infusion bags. Novolin R can be used with the following infusion fluids: 0.9% sodium chloride, 5% dextrose, or 10% dextrose with 40 mmol/L potassium chloride.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Never use Novolin R if it has become viscous or cloudy; use Novolin R only if it is clear and colorless. Vials should not be used if leakage is observed. Novolin R should not be used after the printed expiration date.
The onset of action of Novolin R, when administered intravenously, is more rapid in comparison to subcutaneous administration.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Administration
Subcutaneous injection of Novolin R should be followed by a meal. Patients should wait approximately 30 minutes after injection before starting the meal [ ]. see Dosage and Administration ( ) 2.2
Any change of insulin dose should be made cautiously and only under medical supervision. Changing from one insulin product to another or changing the insulin strength may result in the need for a change in dosage. As with all insulin preparations, the time course of Novolin R action may vary in different individuals or at different times in the same individual and is dependent on many conditions, including dosage, the site of injection, local blood supply, temperature, and physical activity. Patients who change their level of physical activity or meal plan may require adjustment of insulin dosages. Insulin requirements may be altered during illness, emotional disturbances, or other stresses.
5.2 Hypoglycemia
Hypoglycemia is the most common adverse reaction of all insulin therapies, including Novolin R. Severe hypoglycemia may lead to unconsciousness, convulsions, temporary or permanent impairment of brain function or death. Severe hypoglycemia requiring the assistance of another person, parenteral glucose infusion, and glucagon administration has been observed in clinical trials with insulin, including trials with Novolin R.
The timing of hypoglycemia usually reflects the time-action profile of the administered insulin formulations [ ]. Other factors such as changes in food intake (e.g., amount of food or timing of meals), injection site, exercise, and concomitant medications may also alter the risk of hypoglycemia [ ]. As with all insulins, use caution in patients with hypoglycemia unawareness and in patients who may be predisposed to hypoglycemia (e.g., patients who are fasting or have erratic food intake, pediatric patients, and the elderly). The patient’s ability to concentrate and react may be impaired as a result of hypoglycemia. This may present a risk in situations where these abilities are especially important, such as driving or operating other machinery. see Clinical Pharmacology ( , ) 12.212.3see Drug Interactions ( ) 7
Rapid changes in serum glucose concentrations may induce symptoms of hypoglycemia in patients with diabetes, regardless of the glucose value. Early warning symptoms of hypoglycemia may be different or less pronounced under certain conditions, such as longstanding diabetes, diabetic neuropathy, use of medications such as beta-blockers, or intensified glycemic control [ ]. These situations may result in severe hypoglycemia (and, possibly, loss of consciousness) prior to the patient’s awareness of hypoglycemia. Intravenously administered insulin has a more rapid onset of action than subcutaneously administered insulin, requiring more close monitoring for hypoglycemia. see Drug Interactions ( ) 7
5.3 Hypokalemia
All insulins, including Novolin R, cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia that, if left untreated, may cause respiratory paralysis, ventricular arrhythmia, and death. Use caution in patients who may be at risk for hypokalemia (e.g., patients using potassium-lowering medications and patients taking medications sensitive to serum potassium concentrations). Monitor glucose and potassium frequently when Novolin R is administered intravenously.
5.4 Hyperglycemia, Diabetic Ketoacidosis, and Hyperosmolar Hyperglycemic Non-Ketotic Syndrome
Hyperglycemia, diabetic ketoacidosis, or hyperosmolar hyperglycemic non-ketotic syndrome may develop in patients who take less insulin than needed to control blood glucose. These conditions can be precipitated by illness, infection, dietary indiscretion, or omission or improper administration of the prescribed insulin dose.
5.5 Renal or Hepatic Impairment
As with other insulins, the dose requirements for Novolin R may be reduced in patients with renal or hepatic impairment.
5.6 Hypersensitivity and Allergic Reactions
- As with other insulins, patients may experience redness, swelling, or itching at the site of injection of Novolin R. These reactions usually resolve in a few days to a few weeks, but in some occasions, may require discontinuation of Novolin R. In some instances, these reactions may be related to factors other than insulin, such as irritants in a skin cleansing agent or poor injection technique. Localized reactions and generalized myalgias have been reported with the use of meta-cresol, which is an excipient in Novolin R. Local Reactions
- Severe, life-threatening, generalized allergy, including anaphylaxis may occur with any insulin, including Novolin R. Generalized allergy to insulin may manifest as a whole body rash (including pruritus), dyspnea, wheezing, hypotension tachycardia, or diaphoresis. Systemic Reactions,
5.7 Mixing of Insulins
If Novolin R is mixed with NPH human insulin, Novolin R should be drawn into the syringe first and the mixture should be injected immediately after mixing. Insulin mixtures should not be administered intravenously.
5.8 Antibody Production
Increases in titers of anti-insulin antibodies that react with human insulin have been observed in patients treated with Novolin R. Data from a 12-month controlled trial in patients with type 1 diabetes suggest that the increase in these antibodies is transient. The clinical significance of these antibodies is not known but does not appear to cause deterioration in glycemic control or necessitate increases in insulin dose.
5.9 Fluid retention and heart failure with concomitant use of PPAR-gamma agonists
Thiazolidinediones (TZDs), which are peroxisome proliferator-activated receptor (PPAR)-gamma agonists, can cause dose-related fluid retention, particularly when used in combination with insulin. Fluid retention may lead to or exacerbate heart failure. Patients treated with insulin, including NOVOLIN R, and a PPAR-gamma agonist should be observed for signs and symptoms of heart failure. If heart failure develops, it should be managed according to current standards of care, and discontinuation or dose reduction of the PPAR-gamma agonist must be considered.
-
6 ADVERSE REACTIONS
- Hypoglycemia
Hypoglycemia is the most commonly observed adverse reaction in patients using insulin, including Novolin R [ ]. see Warnings and Precautions ( ) 5.2
- Insulin initiation and glucose control intensification
Intensification or rapid improvement in glucose control has been associated with a transitory, reversible ophthalmologic refraction disorder, worsening of diabetic retinopathy, and acute painful peripheral neuropathy. Over the long-term, improved glycemic control decreases the risk of diabetic retinopathy and neuropathy.
- Lipodystrophy
Long-term use of insulin, including Novolin R, can cause lipodystrophy at the site of repeated insulin injections. Lipodystrophy includes lipohypertrophy (thickening of adipose tissue) and lipoatrophy (thinning of adipose tissue), and may affect insulin absorption. Rotate insulin injection sites within the same region to reduce the risk of lipodystrophy.
- Weight gain
Weight gain can occur with insulin therapies, including Novolin R, and has been attributed to the anabolic effects of insulin and the decrease in glucosuria.
- Peripheral edema
Insulin may cause sodium retention and edema, particularly if previously poor metabolic control is improved by intensified insulin therapy. These symptoms are usually transitory.
- Allergic reactions
As with other insulins, Novolin R can cause injection site reactions. Severe, life-threatening, generalized allergy, including anaphylaxis may occur with any insulin, including Novolin R [ ]. see Warnings and Precautions ( ) 5.6
Clinical Trial Experience
Because clinical trials are conducted under widely varying designs, the adverse reaction rates reported in one clinical trial may not be easily compared to those rates reported in another clinical trial, and may not reflect the rates actually observed in clinical practice.
Adults with type 1 or type 2 diabetes
The incidence of adverse reactions during clinical trials comparing Novolin R and insulin aspart in adults with type 1 diabetes mellitus and type 2 diabetes mellitus are listed in the tables below.
Table 1: Adverse Reactions in a 24-Week Trial Comparing Novolin R and Insulin Aspart in Adults with Type 1 Diabetes Mellitus Also Treated with NPH Insulin (adverse reactions with an incidence ≥ 5% in the Novolin R treatment group are listed)
Novolin R + NPH
N= 286Insulin aspart + NPH
N=596- * Hypoglycemia was defined as an episode of blood glucose concentration <45 mg/dL, with or without symptoms.
%
%
Hypoglycemia *
72
75
Table 2: Adverse Reactions in a 24-Week Trial Comparing Novolin R and Insulin Aspart in Adults with Type 2 Diabetes Mellitus Also Treated with NPH Insulin (adverse reactions with an incidence ≥ 5% in the Novolin R treatment group are listed)
Novolin R + NPH
N= 91Insulin aspart + NPH
N= 91(%) (%) - * Hypoglycemia was defined as an episode of blood glucose concentration <45 mg/dL, with or without symptoms.
Hypoglycemia *
36
27
Children and adolescents with type 1 diabetes
The incidence of adverse reactions during a 24-week clinical trial comparing Novolin R and insulin aspart in children and adolescents with type 1 diabetes mellitus are listed in the table below.
Table 3: Adverse Reactions in a 24-Week Trial Comparing Novolin R and Insulin Aspart in Children and Adolescents with Type 1 Diabetes Mellitus Also Treated with NPH Insulin (adverse reactions with an incidence ≥5% in the Novolin R treatment group are listed)
Novolin R + NPH
N= 96Insulin aspart + NPH
N= 187(%) (%) - * Hypoglycemia was defined as an episode of blood glucose concentration <50 mg/dL, with or without symptoms.
Hypoglycemia *
85
79
Injection site hypertrophy
8
8
Severe Hypoglycemia
Hypoglycemia is the most commonly observed adverse reaction in patients using insulin, including Novolin R Tables 4 and 5 summarize the incidence of severe hypoglycemia in the Novolin R clinical trials. Severe hypoglycemia was defined as hypoglycemia associated with central nervous system symptoms and requiring intervention of another person or hospitalization. The rates of severe hypoglycemia in the Novolin R clinical trials (see Section 14 for a description of the study designs) were comparable for all treatment regimens (see Tables 4 and 5). [See Warnings and Precautions ( )]. 5.3
Table 4: Severe Hypoglycemia in Patients with Type 1 Diabetes
Type 1 Diabetes
Adults
24 weeks in combination with NPH insulin
Type 1 Diabetes
Children and Adolescents
(age 6-18)
24 weeks in combination with NPH insulin
Type 1 Diabetes
Children
(age 2-6)
24 weeks in combination with NPH insulin
Novolin R
Insulin aspart
Novolin R
Insulin aspart
Novolin R
Insulin aspart
Percent of patients (n/total N)
19
(55/286)
18
(105/596)
9
(9/96)
6
(11/187)
12
(3/25)
8
(2/26)
Event/patient/
year
1.1
0.9
0.3
0.2
0.5
0.3
Table 5: Severe Hypoglycemia in Patients with Type 2 Diabetes
Type 2 Diabetes
Adults
24 weeks in combination with
NPH insulin
Novolin R
Insulin aspart
Percent of patients
(n/total N)
5
(5/91)
10
(9/91)
Event/patient/
year
0.2
0.3
-
7 DRUG INTERACTIONS
A number of medications affect glucose metabolism that may require insulin dose adjustment and particularly close monitoring for hypoglycemia or worsening glycemic control.
- The following are examples of medications that may increase the blood glucose-lowering effect of insulin and increase susceptibility to hypoglycemia: oral antidiabetic medications, pramlintide acetate, angiotensin converting enzyme (ACE) inhibitors, disopyramide, fibrates, fluoxetine, monoamine oxidase (MAO) inhibitors, propoxyphene, salicylates, somatostatin analogs (e.g., octreotide), and sulfonamide antibiotics.
- The following are examples of medications that may reduce the blood glucose-lowering effect of insulin, leading to worsening of glycemic control: corticosteroids, niacin, danazol, diuretics, sympathomimetic agents (e.g., epinephrine, salbutamol, terbutaline), isoniazid, phenothiazine derivatives, somatropin, thyroid hormones, estrogens, progestogens (e.g., in oral contraceptives), and atypical antipsychotics.
- Beta-blockers, clonidine, and lithium salts may either potentiate or weaken the blood glucose-lowering effect of insulin.
- Alcohol can increase susceptibility to hypoglycemia.
- Pentamidine may cause hypoglycemia, which may sometimes be followed by hyperglycemia.
- The signs of hypoglycemia may be reduced or absent in patients taking sympatholytic medications such as beta-blockers, clonidine, guanethidine, and reserpine.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B: All pregnancies have a background risk of birth defects, loss, or other adverse outcome regardless of drug exposure. This background risk is increased in pregnancies complicated by hyperglycemia and may be decreased with good glycemic control. It is essential for patients with diabetes or a history of gestational diabetes to maintain good glycemic control before conception and throughout pregnancy. Insulin requirements may decrease during the first trimester, generally increase during the second and third trimesters, and rapidly decline after delivery. Careful monitoring of glucose control is important during pregnancy in patients with diabetes. Therefore, women should be advised to tell their healthcare provider if they intend to become, or if they become, pregnant while taking Novolin R.
No reproductive toxicity studies have been performed with Novolin R.
8.3 Nursing Mothers
It is unknown whether Novolin R is excreted in breast milk. Small amounts of human insulin are secreted into breast milk, the significance of which is not known. Use of Novolin R is compatible with breastfeeding, but insulin doses may need to be adjusted because lactation can reduce insulin requirements.
8.4 Pediatric Use
The safety and effectiveness of subcutaneous injections of Novolin R have been established in pediatric patients (ages 2 to18 years) with type 1 diabetes . Novolin R has not been studied in pediatric patients younger than 2 years of age. Novolin R has not been studied in pediatric patients with type 2 diabetes. [see Clinical Studies ( )] 14.3
In general, pediatric patients with type 1 diabetes are more susceptible to hypoglycemia than adult patients with type 1 diabetes. As in adults, the dosage of Novolin R must be individualized in pediatric patients based on metabolic needs and frequent monitoring of blood glucose . [see Dosage and Administration ( ) and Warnings and Precautions ( )] 2.15.2
8.5 Geriatric Use
In 3 controlled clinical trials 18 of 1285 patients (1.4%) with type 1 diabetes treated with Novolin R and insulin aspart were ≥65 years of age. In 4 controlled clinical trials 151 of 635 patients (24%) with type 2 diabetes were ≥65 years of age. Therefore, conclusions are limited regarding the efficacy and safety of Novolin R in patients ≥65 years of age, particularly in patients with type 1 diabetes. Pharmacokinetic/pharmacodynamic studies to assess the effect of age on Novolin R have not been performed.
Use caution in patients with advanced age, due to the potential for decreased renal function in this population . [see Warnings and Precautions ( and )] 5.25.5
-
10 OVERDOSAGE
Excess insulin administration may cause hypoglycemia and, particularly when given intravenously, hypokalemia. Mild episodes of hypoglycemia usually can be treated with oral glucose. Adjustments in drug dosage, meal patterns, or exercise may be needed. More severe episodes with coma, seizure, or neurologic impairment can be treated with intramuscular or subcutaneous glucagon or intravenous glucose. Sustained carbohydrate intake and observation may be necessary because hypoglycemia may recur after apparent clinical recovery. Hypokalemia must be corrected appropriately. [ see Warnings and Precautions ( , )] 5.25.3
-
11 DESCRIPTION
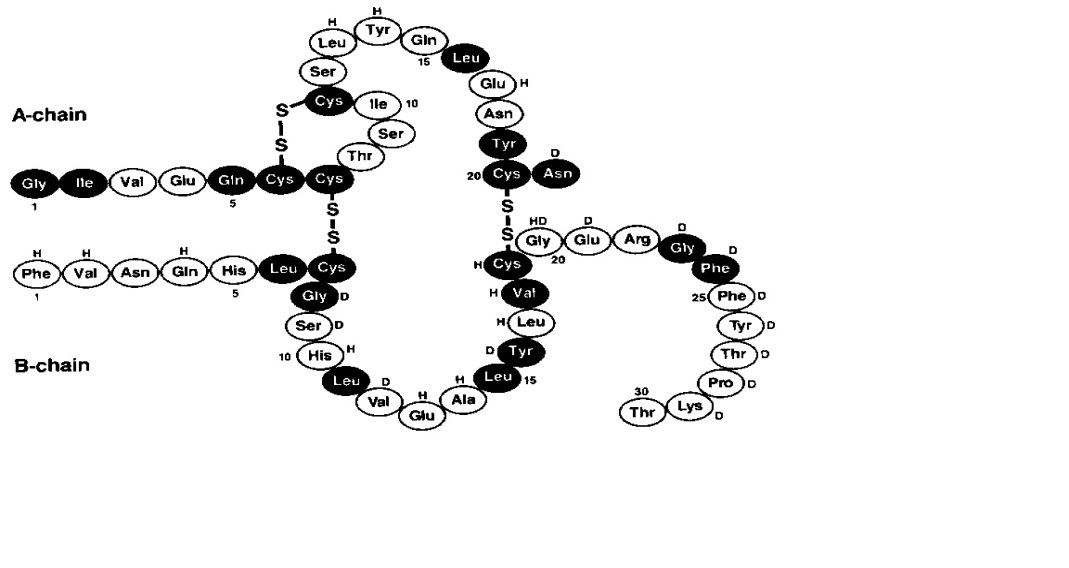
Novolin R (Regular Human Insulin Injection [Recombinant DNA origin] United States Pharmacopeia) is a polypeptide hormone structurally identical to native human insulin and is produced by recombinant DNA technology, utilizing (baker’s yeast) as the production organism. Novolin R has the empirical formula C H N O S and a molecular weight of 5808. Saccharomyces cerevisiae25738365776
Figure 1: Structural formula of Novolin R
Novolin R is a sterile, clear, aqueous, and colorless solution that contains human insulin (rDNA origin) 100 units/mL, glycerol 16 mg/mL, metacresol 3 mg/mL, zinc chloride approximately 7 mcg/mL and water for injection. The pH is adjusted to 7.4. Hydrochloric acid 2N or sodium hydroxide 2N may be added to adjust pH. Novolin R vials are latex-free.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The primary activity of Novolin R is the regulation of glucose metabolism. Insulins, including Novolin R, bind to insulin receptors on muscle and adipocytes and lower blood glucose by facilitating the cellular uptake of glucose and simultaneously inhibiting the output of glucose from the liver.
12.2 Pharmacodynamics
Novolin R is a short-acting insulin. When injected subcutaneously, the glucose-lowering effect of Novolin R begins approximately 30 minutes post-dose, is maximal between 1.5 and 3.5 hours post-dose and terminates approximately 8 hours post-dose. The onset of action of Novolin R, when administered intravenously, is more rapid in comparison to the subcutaneous administration. When injected subcutaneously, Novolin R has a slower onset of action and longer duration of action compared to the rapid-acting insulin analogs.
12.3 Pharmacokinetics
After single subcutaneous administration of 0.1 unit/kg of Novolin R to healthy subjects, peak insulin concentrations occurred between 1.5 to 2.5 hours post-dose. On average, insulin concentrations returned to baseline at around 5 hours post-dose.
The effects of sex, age, obesity, ethnic origin, renal and hepatic impairment, pregnancy, and smoking, on the pharmacodynamics and pharmacokinetics of Novolin R have not been studied.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Standard 2-year carcinogenicity studies in animals have not been performed to evaluate the carcinogenic potential of Novolin R.
Novolin R is not mutagenic in the following tests: The chromosomal aberration assay in human lymphocytes, the micronucleus assay in mouse polychromatic erythrocytes, and the mutation frequency assay in Chinese hamster cells. in vitro
Standard reproduction and teratology studies in animals, including fertility assessments have not been conducted with Novolin R.
-
14 CLINICAL STUDIES
Please see for information on the pharmacokinetics and pharmacodynamics of Novolin R. Section 12 CLINICAL PHARMACOLOGY
14.1 Type 1 diabetes mellitus (adults)
Two six‑month, open‑label, active-controlled studies were conducted to compare the safety and efficacy of Novolin R and insulin aspart in adults with type 1 diabetes. Insulin aspart was administered by subcutaneous injection immediately prior to meals and Novolin R was administered by subcutaneous injection 30 minutes before meals. Both treatment groups also received subcutaneous injections of NPH insulin in either single or divided daily doses. Because the two study designs and results were similar, data are shown for only one study (see Table 6)
Table 6: Subcutaneous Novolin R Administration in Type 1 Diabetes (24 weeks; N=882)
- * Values are Mean ± SD
Novolin R + NPH
N=286
Insulin aspart + NPH N=596
Baseline HbA1c (%) *
8.0 ± 1.2
7.9 ±1.1
Change from Baseline HbA (%) 1c*
0.0 ± 0.8
-0.1 ± 0.8
Treatment Difference in HbA , Mean (95% confidence interval) 1c
Novolin R – insulin aspart group
0.2 [0.1; 0.3]
Baseline, total insulin dose (units/kg/day) *
0.7 ± 0.2
0.7 ± 0.2
End-of-Study, total insulin dose (units/kg/day) *
0.7 ± 0.2
0.7 ± 0.2
Baseline body weight (kg) *
Weight Change from baseline (kg) *
75.9 ± 13.1
0.9 ± 2.9
75.3 ± 14.5
0.5 ± 3.3
14.2 Type 2 diabetes mellitus (adults)
A six‑month, open‑label, active-controlled study was conducted to compare the safety and efficacy of Novolin R and insulin aspart in adults with type 2 diabetes (Table 7). Insulin aspart was administered by subcutaneous injection immediately prior to meals and Novolin R was administered by subcutaneous injection 30 minutes before meals. Both treatment groups also received subcutaneous injections of NPH insulin in either single or divided daily doses.
Table 7: Subcutaneous Novolin R Administration in Type 2 Diabetes (24 weeks; N=182)
- * Values are Mean ± SD
Novolin R + NPH
N = 91
Insulin aspart + NPH
N = 91
Baseline HbA (%) 1c*
7.8 ± 1.1
8.1 ± 1.2
Change from Baseline HbA (%) 1c*
-0.1 ± 0.8
-0.3 ± 1.0
Treatment Difference in HbA Mean (95% confidence interval) 1c,
Novolin R – insulin aspart group
0.1 [-0.1; 0.4]
Baseline, total insulin dose (units/kg/day) *
0.6 ± 0.3
0.6 ± 0.3
End-of-Study, total insulin dose (units/kg/day) *
0.7 ± 0.3
0.7 ± 0.3
Baseline body weight (kg) *
Weight Change from baseline (kg) *
85.8 ± 14.8
0.4 ± 3.1
88.4± 13.3
1.2± 3.0
14.3 Type 1 diabetes mellitus (children and adolescents)
A six‑month, open‑label, active-controlled study was conducted to compare the safety and efficacy of Novolin R and insulin aspart in children and adolescents aged 6-18 years with type 1 diabetes (Table 8). Insulin aspart was administered by subcutaneous injection immediately prior to meals and Novolin R was administered by subcutaneous injection 30 minutes before meals. Both treatment groups also received subcutaneous injections of NPH insulin.
Table 8: Subcutaneous Novolin R Administration in Children and Adolescents with Type 1 Diabetes (24 weeks; N=283)
- * Values are Mean ± SD
- † The treatment difference and corresponding 95% confidence interval is based on the Analysis of Covariance Model
Novolin R + NPH
N=96
Insulin aspart + NPH
N=187
Baseline HbA (%) 1c*
8.3 ± 1.3
8.3 ± 1.2
Change from Baseline HbA (%) 1c*
0.1 ± 1.1
0.1 ± 1.0
Treatment Difference in HbA Mean (95% confidence interval) 1c,
Novolin R – insulin aspart group †
0.2 [-0.1; 0.5]
Baseline, total insulin dose (units/kg/day) *
1.0 ± 0.4
1.0 ± 0.3
End-of-Study, total insulin dose (units/kg/day) *
1.2 ± 0.4
1.2 ± 0.4
Diabetic ketoacidosis n (%)
2 (2%)
10 (5%)
Baseline body weight (kg) *
Weight Change from baseline (kg) *
48.7 ± 15.8
2.4 ± 2.6
50.6 ± 19.6
2.7 ± 3.5
Novolin R and insulin aspart have also been compared in an open-label, randomized, crossover trial in 26 children with type 1 diabetes aged 2-6 years. Patients received each treatment for 12 weeks. Insulin aspart was administered by subcutaneous injection immediately prior to meals and Novolin R was administered by subcutaneous injection 30 minutes before meals. Both treatment groups also received subcutaneous injections of NPH insulin. In this study, the mean baseline HbA was 7.8%. 1c
The estimated HbA at end of treatment was 7.6% with Novolin R and 7.7% with insulin aspart. 1c
-
16 HOW SUPPLIED/STORAGE AND HANDLING
NDC: 64725-1833-1 in a VIAL of 10 INJECTION, SOLUTIONS
16.1 How Supplied
Novolin R is available in 10 mL vials (NDC: 0169-1833-11 and ReliOn brand NDC: 0169-1833-02). The concentration of Novolin R is 100 USP units of human insulin (rDNA origin)/mL. One vial is provided in each sale pack. ®
16.2 Recommended Storage
Unopened Novolin R vials should be stored in the refrigerator (36° - 46°F [2° - 8°C]). If carried as a spare or if refrigeration is not possible, unopened Novolin R vials can be kept at room temperature provided they are kept as cool as possible (not above 77 F [25 C]). If kept at room temperature, Novolin R vials must be discarded after 42 days even if they are unopened. oo
In addition, unopened Novolin R vials should be kept in their cartons so that they will stay clean and protected from light. They should not be exposed to heat or light. Do not freeze and do not use Novolin R if it has been frozen.
An opened (In use) Novolin R vial can be kept at room temperature provided it is kept as cool as possible (not above 77 F [25 C]) and away from heat or light. Do not refrigerate after first use. oo
Unopened and opened (In use) Novolin R vials must be discarded 42 days after they are first kept out of the refrigerator, even if they still contain Novolin R insulin.
Table 9: Storage Conditions for Novolin R vials
- * The total time allowed at room temperature (up to 25 C) is 42 days regardless of whether the product is unopened or opened (In use) o
Unopened
(Refrigerated)
Unopened
(Room Temperature up to 77 F [25 C] ) oo
Opened (In use)
(Room Temperature up to 77 F [25 C]) oo
Until expiration date
42 days *
42 days *
Infusion bags prepared as indicated under are stable at room temperature for 24 hours. A certain amount of insulin will be initially adsorbed to the material of the infusion bag. DOSAGE AND ADMINISTRATION ( ) 2.3
Always remove the needle after each injection. Always use a new disposable syringe and needle for each injection to prevent contamination.
Never use insulin after the expiry date which is printed on the label and carton.
-
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling (Patient Information and Instructions for Use)
17.1 Instructions for All Patients
Maintenance of normal or near-normal glucose control is a treatment goal in diabetes mellitus and has been associated with a reduction in some diabetic complications. Patients should be informed about potential risks and benefits of Novolin R therapy including possible adverse reactions. Patients should also be offered continued education and advice on insulin therapies, injection technique, life-style management, regular glucose monitoring, periodic glycosylated hemoglobin testing, recognition and management of hypo- and hyperglycemia, adherence to meal planning, complications of insulin therapy, timing of dose, instruction in the use of injection devices, and proper storage of insulin. Patients should be informed that frequent, patient-performed blood glucose measurements are needed to achieve optimal glycemic control and avoid both hyper- and hypoglycemia.
The patient’s ability to concentrate and react may be impaired as a result of hypoglycemia. This may present a risk in situations where these abilities are especially important, such as driving or operating other machinery. Patients who have frequent hypoglycemia or reduced or absent warning signs of hypoglycemia should be advised to use caution when driving or operating machinery. Female patients should be advised to tell their physician if they intend to become, or if they become pregnant.
Patients should be instructed to always carefully check that they are administering the correct insulin to avoid medication errors between Novolin R and other insulins. Patients should check the label for the drug name Novolin R, the enlarged R letter, and the blue horizontal bar. If a prescription for Novolin R is needed, it should be written clearly to avoid confusion with other insulin products.
Novolin is a registered trademark of Novo Nordisk A/S. ®
ReliOn is a registered trademark of Wal-Mart Stores, Inc. and is used under license by Novo Nordisk Inc. ®
© 2002-2013 Novo Nordisk
Manufactured by:
Novo Nordisk A/S
DK-2880 Bagsvaerd, Denmark
ReliOn brand manufactured by: ®
Novo Nordisk A/S
DK-2880 Bagsvaerd, Denmark
For Wal-Mart Stores Inc.
For information about Novolin R contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, New Jersey 08536
1-800-727-6500
-
PATIENT PACKAGE INSERT
Patient Information
NOVOLIN R (NO-voe-lin) ®
(Regular, Human Insulin Injection [recombinant DNA origin] USP)
solution for subcutaneous injection
Read the Patient Information leaflet that comes with Novolin R before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your healthcare provider about your diabetes or your treatment. Make sure you know how to manage your diabetes. Ask your healthcare provider if you have any questions about managing your diabetes. ®
What is Novolin R? ®
Novolin R is a man-made insulin (recombinant DNA origin) that is used to control high blood sugar in adults and children with diabetes mellitus. ®
Who should not use Novolin R? ®
Do not take Novolin R if: ®
- Your blood sugar is too low (hypoglycemia). After treating your low blood sugar, follow your healthcare provider’s instructions on the use of Novolin R. ®
- You are allergic to any of the ingredients in Novolin R. See the end of this leaflet for a complete list of ingredients in Novolin R. Check with your healthcare provider if you are not sure. ®®
What should I tell my healthcare provider before taking Novolin R? ®
Before you take Novolin R, tell your healthcare providers if you: ®
- have liver or kidney problems.
- take any other medicines, especially ones commonly called TZDs (thiazolidinediones).
- If you have heart failure, it may get worse while you take TZDs with Novolin R. have heart failure or other heart problems.®
- Medical conditions can affect your insulin needs and your dose of Novolin R. have any other medical conditions.®
- Talk to your healthcare provider if you are pregnant or plan to become pregnant. You and your healthcare provider should talk about the best way to manage your diabetes while you are pregnant. are pregnant or plan to become pregnant.
- It is not known if Novolin R passes into breast milk. You and your healthcare provider should decide if you will take Novolin R while you breast-feed. are breast-feeding or plan to breast-feed.®®
including prescription and nonprescription medicines, vitamins and herbal supplements. Novolin R may affect the way Tell your healthcare provider about all of the medicines you take,®
other medicines work, and other medicines may affect how Novolin R works. ®
Keep a list of your medicines with you to show all your Know the medicines you take.
healthcare providers and pharmacist when you get a new medicine.
How should I take Novolin R? ®
- Novolin R comes in 10 mL vials for use with a syringe. ®
- Take Novolin R exactly as prescribed. ®
- Your healthcare provider will tell you how much Novolin R to take and when to take it. ®
- Do not make any changes to your dose or type of insulin unless you are told to do so by your healthcare provider.
- The effects of Novolin R usually start working within about 30 minutes after your injection and usually lasts for up to 8 hours. ®
- your total dose of insulin, your dose of Novolin R, your dose of longer-acting insulin, or the number of injections of insulin you use. While using Novolin R your healthcare provider may change ®®
- with any insulins other than NPH in the same syringe. Do not mix Novolin R ®
- Novolin R may affect your blood sugar levels faster if you inject it into the skin of your abdomen (stomach area). Inject Novolin R under your skin (subcutaneously) of your abdomen (stomach area), upper arms, buttocks or upper legs. ®®Never inject Novolin R into a vein or into a muscle. ®
- Do not use Novolin R in an insulin pump. ®
- Change (rotate) your injection site within the chosen area (for example, stomach or upper arm) with each dose. Do not inject into the same spot for each injection.
- Talk to your healthcare provider if you have any questions. Your healthcare provider should show you how to inject Novolin R before you start taking it. Read the instructions for use that comes with your Novolin R. ®®
- You can treat mild low blood sugar (hypoglycemia) by drinking or eating something sugary right away (fruit juice, sugar candies, or glucose tablets). It is important to treat low blood sugar (hypoglycemia) right away because it could get worse and could lead to passing out (loss of consciousness), seizures and death. If you take too much Novolin R, your blood sugar may fall too low (hypoglycemia). ®
-
If high blood sugar (hyperglycemia) is not treated it can lead to serious problems, like loss of consciousness (passing out), coma or even death. Follow your healthcare provider’s instructions for treating high blood sugar. Know your symptoms of high blood sugar which may include:
If you forget to take your dose of Novolin R, your blood sugar may go too high (hyperglycemia).
®
- ∘ increased thirst
- ∘ frequent urination and dehydration
- ∘ confusion or drowsiness
- ∘ loss of appetite
- ∘ fruity smell on breath
- ∘ high amounts of sugar and ketones in your urine
- ∘ nausea, vomiting (throwing up) or stomach pain
- ∘ a hard time breathing
- Do not share needles or syringes with others. You may give an infection to them or get an infection from them.
- Ask your healthcare provider what your blood sugars should be and how often you should check your blood sugar levels for hypoglycemia (too low blood sugar) and hyperglycemia (too high blood sugar). Check your blood sugar levels.
Your insulin dosage may need to change because of:
- illness
- stress
- other medicines you take
- change in diet
- change in physical activity or exercise
- surgery
See the end of this patient information for instructions about preparing and giving the injection.
What should I avoid while taking Novolin R? ®
-
Alcohol may affect your blood sugar when you take Novolin R. This could lead to blood sugar that is too low (hypoglycemia).
Drinking alcohol.®
- ∘ You may have trouble paying attention or reacting if you have low blood sugar (hypoglycemia). Be careful when you drive a car or operate machinery. Ask your healthcare provider if it is alright for you to drive if you often have: Driving and operating machinery.
- ∘ low blood sugar
- ∘ decreased or no warning signs of low blood sugar
What are the possible side effects of Novolin R? ®
Novolin R may cause serious side effects, including: ®
- Low blood sugar (hypoglycemia).
The general symptoms of low blood sugar (hypoglycemia) may be one or more of the following:
- sweating
- dizziness or lightheadedness
- shakiness
- hunger
- fast heart beat
- tingling in your hands, feet, lips or tongue
- trouble concentrating or confusion
- blurred vision
- slurred speech
- anxiety, irritability or mood changes
- headache
Very low blood sugar (hypoglycemia) can cause loss of consciousness (passing out), seizures, temporary or permanent brain problems or death.
Talk to your healthcare provider about how to tell if you have low blood sugar and what to do if this happens while taking Novolin R. Know your symptoms of low blood sugar. Follow your healthcare provider’s instructions for treating low blood sugar. ®
Talk to your healthcare provider if low blood sugar is a problem for you. Your dose of Novolin R may need to be changed. ®
- A decrease of potassium in your blood can cause breathing problems, a change in your heartbeat and death. Low blood potassium (hypokalemia).
-
Serious allergic reaction (whole body reaction). You can have a serious allergic reaction that may be life-threatening. Get medical help right away if you have any of these symptoms of an allergic reaction:
- a rash over your body
- have trouble breathing
- a fast heartbeat
- sweating
- feel faint
- Swelling of your hands and feet.
-
Taking certain diabetes pills called thiazolidinediones or “TZDs” with Novolin R may cause heart failure in some people. This can happen even if you have never had heart failure or heart problems before. If you already have heart failure it may get worse while you take TZDs with Novolin R . Your healthcare provider should monitor you closely while you are taking TZDs with Novolin R. Tell your healthcare provider if you have any new or worse symptoms of heart failure including:
Heart Failure.®®®
- shortness of breath
- swelling of your ankles or feet
- sudden weight gain
- Treatment with TZDs and Novolin R may need to be adjusted or stopped by your healthcare provider if you have new or worse heart failure. ®
Other side effects of Novolin R may include: ®
- You may get redness, swelling, and itching at the injection site. If you keep having skin reactions, or they are serious, talk to your healthcare provider. You may need to stop using Novolin R and use a different insulin. Do not inject insulin into skin that is red, swollen, or itchy. Reactions at the injection site (local allergic reaction).®
- The fatty tissue under the skin may shrink (lipoatrophy) or thicken (lipohypertrophy) at the injection site. Change (rotate) the site where you inject your insulin to help reduce the chance of developing these skin changes. Do not inject insulin into this type of skin. Changes at the injection site (lipodystrophy).
- Weight gain.
- Swelling of your arms and legs.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects from Novolin R. Ask your healthcare provider or pharmacist for more information. ®
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Novolin R? ®
Unopened Novolin R: ®
- Unopened vials can be used until the expiration date on the Novolin R label, if the medicine has been stored in a refrigerator. Unopened Novolin R should be kept in the refrigerator between 36°F to 46°F (2° to 8°C). ®®
- If refrigeration is not possible or if you want to carry a spare Novolin R vial you can keep the unopened vial at room temperature for up to 42 days, as long as it is kept at or below 77°F (25°C). Throw away the vial 42 days after it is first kept out of the refrigerator, even if the vial is unopened. ®
- Do not freeze. Do not use Novolin R if it has been frozen. ®
- Keep unopened Novolin R in the carton to protect it from light. ®
Novolin R in use: ®
- Keep at room temperature below 77°F (25°C).
- Keep vials away from heat or light.
- Do not refrigerate an opened vial.
- Throw away the vial 42 days after it is first kept out of the refrigerator, even if there is insulin left in the vial.
Never use insulin after the expiration date which is printed on the label and carton.
General information about Novolin R ®
Medicines are sometimes prescribed for conditions that are not mentioned in the patient leaflet. Do not use Novolin R for a condition for which it was not prescribed. Do not give Novolin R to other people, even if they have the same symptoms you have. It may harm them. ®®
This leaflet summarizes the most important information about Novolin R. If you would like more information about Novolin R or diabetes, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about Novolin R that is written for healthcare professionals. ®®®
For more information about Novolin R, call 1-800-727-6500 or go to . ®www.novonordisk-us.com
What are the ingredients in Novolin R? ®
Regular Human Insulin Injection (recombinant DNA origin) USP. Active ingredient:
glycerol, metacresol, zinc chloride, water for injection, hydrochloric acid and sodium hydroxide may be added. Inactive ingredients:
All Novolin R vials are latex-free. ®
This Patient Information has been approved by the U.S. Food and Drug Administration.
Date of issue: 03/2013
Novolin is a registered trademark of Novo Nordisk A/S. ®
© 2002-2013 Novo Nordisk
Manufactured by:
Novo Nordisk A/S
DK-2880 Bagsvaerd, Denmark
For information about Novolin R contact: ®
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, New Jersey 08536
1-800-727-6500
www.novonordisk-us.com
Patient Instructions for Use
Novolin R 10 mL vial (100 Units/mL, U-100) ®
Please read the following Instructions for Use carefully before using your Novolin R 10 mL vial and ®
each time you get a refill. You should read the instructions in this manual even if you have used an
insulin 10 mL vial before. There may be new information.
Before starting, gather all of the supplies that you will need to use for preparing and giving your insulin
injection.
Never re-use syringes and needles.
How should I use the Novolin R vial?
1. Check to make sure that you have the correct type of insulin. This is especially important if you use different types of insulin.
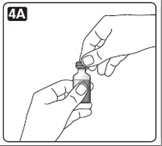
2. Look at the vial and the insulin. The insulin should be clear and colorless. The tamper-resistant cap should be in place before the first use. If the cap had been removed before your first use of the vial, or if the insulin is cloudy, colored, or contains any particles, do not use it and call Novo Nordisk at 1-800-727-6500.
3. Wash your hands with soap and water. Clean your injection site with an alcohol swab and let the injection site dry before you inject. Talk with your healthcare provider about how to rotate injection sites and how to give an injection.
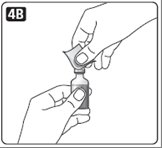
4. If you are using a new vial, pull off the tamper-resistant cap.
Wipe the rubber stopper with an alcohol swab.
5. Do not roll or shake the vial. Shaking right before the dose is drawn into the syringe may cause bubbles or foam. This can cause you to draw up the wrong dose of insulin.
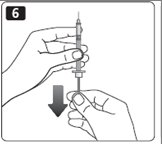
6. Pull back the plunger on the syringe until the black tip reaches the marking for the number of units you will inject.
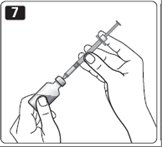
7. Push the needle through the rubber stopper of the vial.
8. Push the plunger all the way in to force air into the vial.
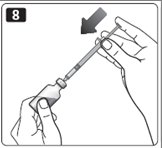
9. Turn the vial and syringe upside down and slowly pull the plunger back to a few units beyond the correct dose.
10. If there are any air bubbles, tap the syringe gently with your finger to raise the air bubbles to the top. Then slowly push the plunger to the marking for your correct dose. This process should move any air bubbles present in the syringe back into the vial.
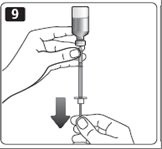
11. Check to make sure you have the right dose of Novolin R in the syringe.
12. Pull the syringe out of the vial’s rubber stopper.
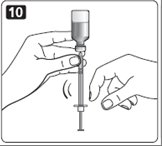
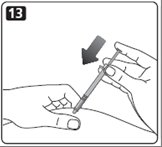
13. Your healthcare provider should tell you if you need to pinch the skin before and while inserting the needle. This can vary from patient to patient so it is important to ask your healthcare provider if you did not receive instructions on pinching the skin. Insert the needle into the skin. Press the plunger of the syringe to inject the insulin. When you are finished injecting the insulin, pull the needle out of your skin. You may see a drop of Novolin R at the needle tip. This is normal and has no effect on the dose you just received. If you see blood after you take the needle out of your skin, press the injection site lightly with a piece of gauze or an alcohol wipe. Do not rub the area.
14. After your injection, do not recap the needle. Place used syringes, needles and used insulin vials in a disposable puncture-resistant sharps container, or some type of hard plastic or metal container with a screw on cap such as a detergent bottle or coffee can.
15. Ask your healthcare provider about the right way to throw away used syringes and needles. There may be state or local laws about the right way to throw away used syringes and needles. Do not throw away used needles and syringes in household trash or recycle.
How should I mix Novolin R with NPH insulin?
Different insulins should be mixed only under instruction from a healthcare provider. Do not mix
Novolin R should be mixed with NPH insulin right before use. When you are mixing Novolin R insulin with NPH insulin, always Novolin R with any other type of insulin except NPH insulin.draw the Novolin R (clear) insulin into the syringe first.
1. Add together the total number of units of NPH and Novolin R that you need to inject. Your total dose of medicine to inject will be the amount of NPH and Novolin R in the syringe after drawing up both insulins. For example, if you need 5 units of NPH and 2 units of Novolin R, the total dose of insulin in
the syringe would be 7 units.
Preparing your NPH and Novolin R insulins for injection:
2. Roll the NPH vial between your hands until all of the liquid in the vial is cloudy.
3. Pull the plunger of the syringe down so that the dark end is lined up to the number of units needed for your NPH insulin. This will draw into the syringe the same amount of air as the NPH dose needed.
4. Put the needle through the rubber stopper of the cloudy NPH insulin bottle. After you inject the air into the NPH vial, remove the needle from the vial but do not withdraw any of the NPH insulin. Putting air in the bottle makes it easier to draw the insulin out of the bottle.
5. Pull the plunger of the syringe down to the number of units needed for your Novolin R insulin. After you draw the air into the syringe, inject the air into the Novolin R vial.
Drawing up and mixing your NPH and Novolin R insulins for injection:
6. With the needle in place, turn the clear insulin vial of Novolin R upside down and slowly pull the plunger back to a few units beyond the right dose of Novolin R. The tip of the needle must be in the Novolin R liquid to get the full dose and not an air dose.
7. Check the syringe for air bubbles. If you see air bubbles, tap the syringe gently with your finger to
raise the air bubbles to the top. Then slowly push the plunger to the marking for your correct dose. This process should move any air bubbles in the syringe back into the vial.
8. After withdrawing the needle from the Novolin R vial, insert the needle into the NPH vial.
9. Turn the NPH vial upside down with the syringe and needle still in the vial. Slowly pull the plunger back to withdraw your NPH dose.
Remember the total dose of medicine in the syringe should be your total dose of NPH and Novolin R insulins. (See Step 1 under “How should I mix Novolin R with NPH insulin?”)
10. Inject your insulin right away otherwise it might not work properly.
This Patient Instructions for Use has been approved by the Food and Drug Administration.
Date of Issue: 03/2013
Novolin is a registered trademark of Novo Nordisk A/S. ®
© 2002-2013 Novo Nordisk
Manufactured by:
Novo Nordisk A/S
DK-2880 Bagsvaerd, Denmark
For information about Novolin R contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, New Jersey 08536
1-800-727-6500
- NOVOLIN R (HUMAN INSULIN) INJECTION, SOLUTION
-
INGREDIENTS AND APPEARANCE
NOVOLIN R
human insulin injection, solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 64725-1833(NDC:0169-1833) Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) INSULIN HUMAN 100 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength ZINC CHLORIDE (UNII: 86Q357L16B) 7 ug in 1 mL GLYCERIN (UNII: PDC6A3C0OX) 16 mg in 1 mL METACRESOL (UNII: GGO4Y809LO) 3 mg in 1 mL HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 64725-1833-1 10 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019938 06/25/1991 Labeler - TYA Pharmaceuticals (938389038) Registrant - TYA Pharmaceuticals (938389038) Establishment Name Address ID/FEI Business Operations TYA Pharmaceuticals 938389038 RELABEL(64725-1833) , REPACK(64725-1833)
Trademark Results [Novolin]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NOVOLIN 73498651 1384926 Live/Registered |
NOVO INDUSTRI A/S 1984-09-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.