NUPRIN IMMEDIATE RELEASE- ibuprofen tablet, coated
NUPRIN by
Drug Labeling and Warnings
NUPRIN by is a Otc medication manufactured, distributed, or labeled by Strides Arcolab Limited, Shasun Pharmaceuticals Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT(S)
- PURPOSE
- USE(S)
-
WARNINGS
Allergy alert
Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin.
Symptoms may include:- hives
- facial swelling
- rash
- blisters
- shock
- skin reddening
- asthma (wheezing)
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning
This product contains a nonsteroidal anti-inflammatory drug (NSAID), which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
- DO NOT USE
-
ASK A DOCTOR BEFORE USE IF
- you have problems or serious side effects from taking pain relievers or fever reducers
- the stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
- you have asthma
- you are taking a diuretic
- ASK A DOCTOR OR PHARMACIST BEFORE USE IF
- WHEN USING THIS PRODUCT
-
STOP USE AND ASK DOCTOR IF
- you experience any of the following signs of stomach bleeding
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3days
- redness or swelling is present in the painful area
- any new symptoms appear
If pregnant or breast-feeding, ask a health professional before use. It is especially important not to use ibuprofen during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
- you experience any of the following signs of stomach bleeding
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
do not take more than directed
the smallest effective dose should be used
adults and children 12 years and older- take 1 tablet every 4 to 6 hours while symptoms persist
- if pain or fever does not respond to 1 tablet, 2 tablets may be used
- do not exceed 6 tablets in 24 hours, unless directed by a doctor
children under 12 years
-
ask a doctor
OTHER INFORMATION- store between 20 – 25°C (68-77°F)
- tamper evident: do not use if imprinted safety seal under cap is broken or missing
- see end panel for lot number and expiration date
-
INACTIVE INGREDIENT (S)
colloidal silicon dioxide, corn starch, hypromellose, magnesium stearate, microcrystalline cellulose, pregelatinized starch, sodium starch glycolate, talc, titanium dioxide and triacetin
QUESTIONS OR COMMENTS?
Adverse drug event Call (877)-244-9825
MADE IN INDIAMfg.Lic.No. DRUGS/PY/05 13 1523
NUPRIN® is a registered trademark of SVADS Holdings SA
Manufactured for:
Strides Pharma Inc,
East Brunswick, NJ 08816
www.nuprin.com
December 2015 -
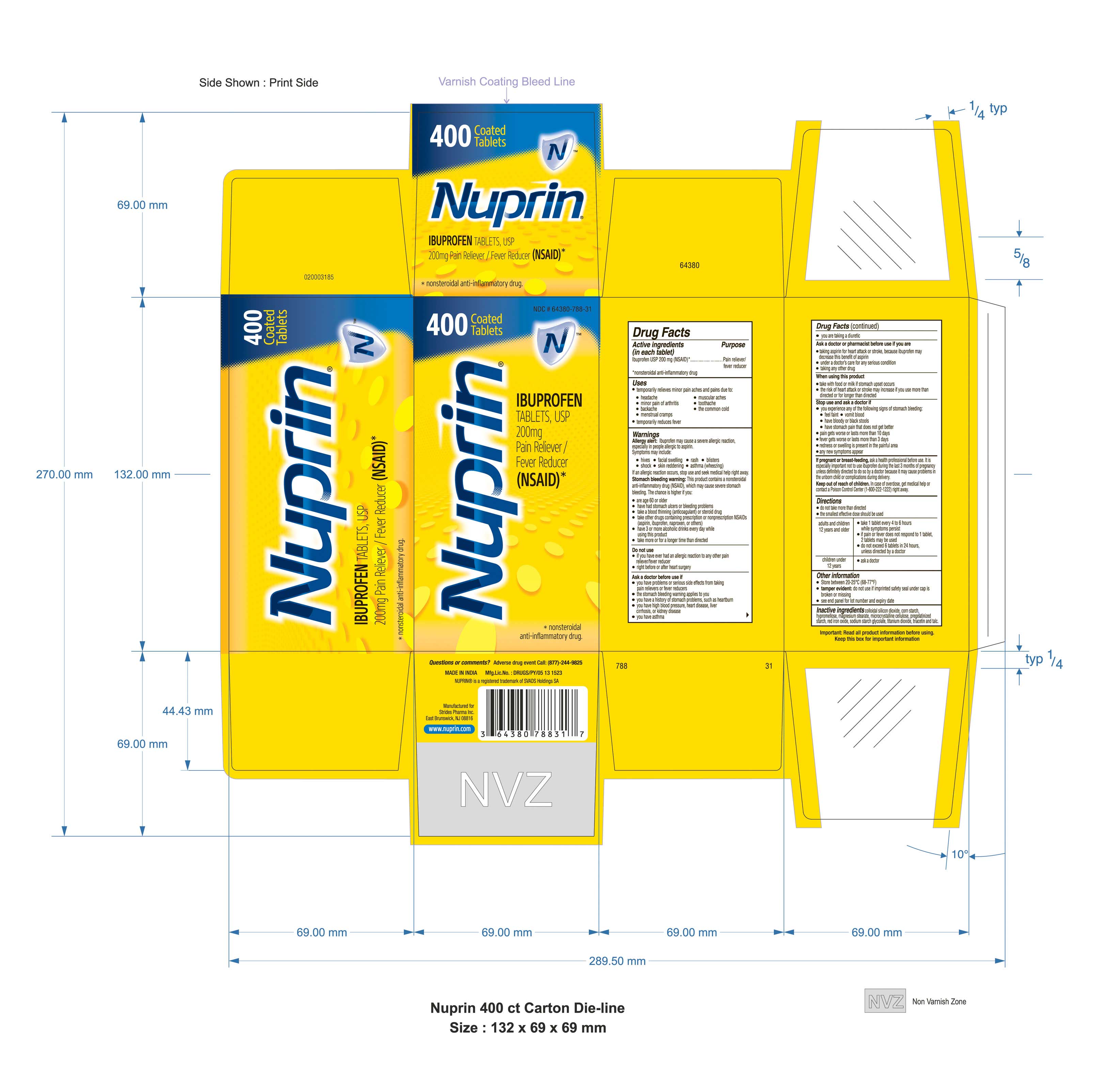
PRINCIPAL DISPLAY PANEL
Package Label (Round Shaped Tablets) - Principal Display Panel, 200 mg Tablets
NDC: 64380-788-31
400 Coated Tablets
N TMNuprin®
IBUPROFEN TABLETS,USP
200 mg
Pain Reliever/Fever Reducer (NSAID)*
*nonsteroidal anti-inflammatory drug.

-
INGREDIENTS AND APPEARANCE
NUPRIN IMMEDIATE RELEASE
ibuprofen tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 64380-788 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) HYPROMELLOSES (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color BROWN Score no score Shape ROUND (ROUND SHAPED) Size 10mm Flavor Imprint Code IBU200 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 64380-788-31 1 in 1 CARTON 1 400 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA079129 12/16/2015 Labeler - Strides Arcolab Limited (650738743) Registrant - Shasun Pharmaceuticals Limited (915786829) Establishment Name Address ID/FEI Business Operations Shasun Pharmaceuticals Limited 915786829 MANUFACTURE(64380-788)
Trademark Results [NUPRIN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NUPRIN 88249859 not registered Live/Pending |
Strides Global Consumer Healthcare Limited 2019-01-04 |
 NUPRIN 87384264 not registered Live/Pending |
Strides Global Consumer Healthcare Limited 2017-03-24 |
 NUPRIN 86755380 not registered Dead/Abandoned |
SVADS Holding SA 2015-09-14 |
 NUPRIN 86561952 not registered Dead/Abandoned |
SVADS Holding SA 2015-03-12 |
 NUPRIN 86561938 not registered Dead/Abandoned |
SVADS Holding SA 2015-03-12 |
 NUPRIN 86302645 not registered Dead/Abandoned |
STRIDES GLOBAL CONSUMER HEALTHCARE LIMITED 2014-06-06 |
 NUPRIN 85917705 not registered Dead/Abandoned |
SOBEK THERAPEUTICS, LLC 2013-04-29 |
 NUPRIN 78717745 3226852 Live/Registered |
STRIDES GLOBAL CONSUMER HEALTHCARE LIMITED 2005-09-21 |
 NUPRIN 76642938 not registered Dead/Abandoned |
LFB HOLDINGS, INC. 2005-07-15 |
 NUPRIN 73423663 1301164 Dead/Cancelled |
Upjohn Company, The 1983-04-28 |
 NUPRIN 72122662 0731407 Dead/Expired |
UPJOHN COMPANY, THE 1961-06-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.