HEVERT DETOX- arsenic trioxide, clematis recta flowering top, conium maculatum flowering top, lachesis muta venom, mercuric iodide, phytolacca americana root, toxicodendron pubescens shoot, scrophularia nodosa leaf with stem, sulfur, milk thistle, chelidonium majus root, taraxacum officinale, nitric acid, apis mellifera, atropa belladonna, lytta vesicatoria, juniper berry, solidago virgaurea flowering top, eichhornia crassipes whole, okoubaka aubrevillei bark, quassia amara wood, and taraxacum officinale kit
HEVERT DETOX by
Drug Labeling and Warnings
HEVERT DETOX by is a Homeopathic medication manufactured, distributed, or labeled by Hevert Pharmaceuticals LLC, Hevert Arzneimittel GmbH & Co. KG, Q Pharma Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
ACTIVE INGREDIENT
Active ingredients* Purpose - * "X" indicates a homeopathic dilution.
For more information visit: www.hevertusa.com- † Relief of discomfort associated with lymphatic swelling and edema
Arsenicum album 6X † Clematis 4X † Conium 6X † Lachesis 8X † Mercurius bijodatus 8X † Phytolacca 4X † Rhus toxicodendron 6X † Scrophularia nodosa, herba 3X † Sulfur 4X † - * "X" indicates a homeopathic dilution.
-
Uses1
For temporary relief of discomfort associated with lymphatic swelling and edema.
- 1 Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
-
Warnings
Chronically enlarged lymph nodes can be the sign of different diseases. Therefore, consult a doctor in order to clarify the underlying disease before using this medicine. Also consult a doctor promptly in case of acute signs of inflammation (redness, heat, swelling, pain and dysfunction) and fever or enlargement of the lymph nodes during treatment.
Do not use if
- you are allergic to Rhus toxicodendron (poison ivy) or other plants from the cashew family
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses2
For temporary relief of liver and gallbladder driven symptoms of indigestion, such as:
- bloating
- pressure and pain in upper abdomen
- constipation.
- 2 Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
-
Warnings
Gallstones, occlusion of the bile ducts, jaundice and any other unclear or persistent discomfort in the upper abdomen may be serious. Consult a doctor promptly if any of these symptoms occur.
- Additional Warnings
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses3
For temporary relief of pain and burning sensation associated with frequent or urgent need to urinate.
- 3 Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
- Warnings
- Additional Warnings
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses4
For temporary relief of symptoms of upset stomach and indigestion, such as:
bloating, abdominal fullness, nausea and constipation.
- 4 Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
- Warnings
- Additional Warnings
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
- Warnings
- Directions
- Other Information
- Questions?
- SPL UNCLASSIFIED SECTION
-
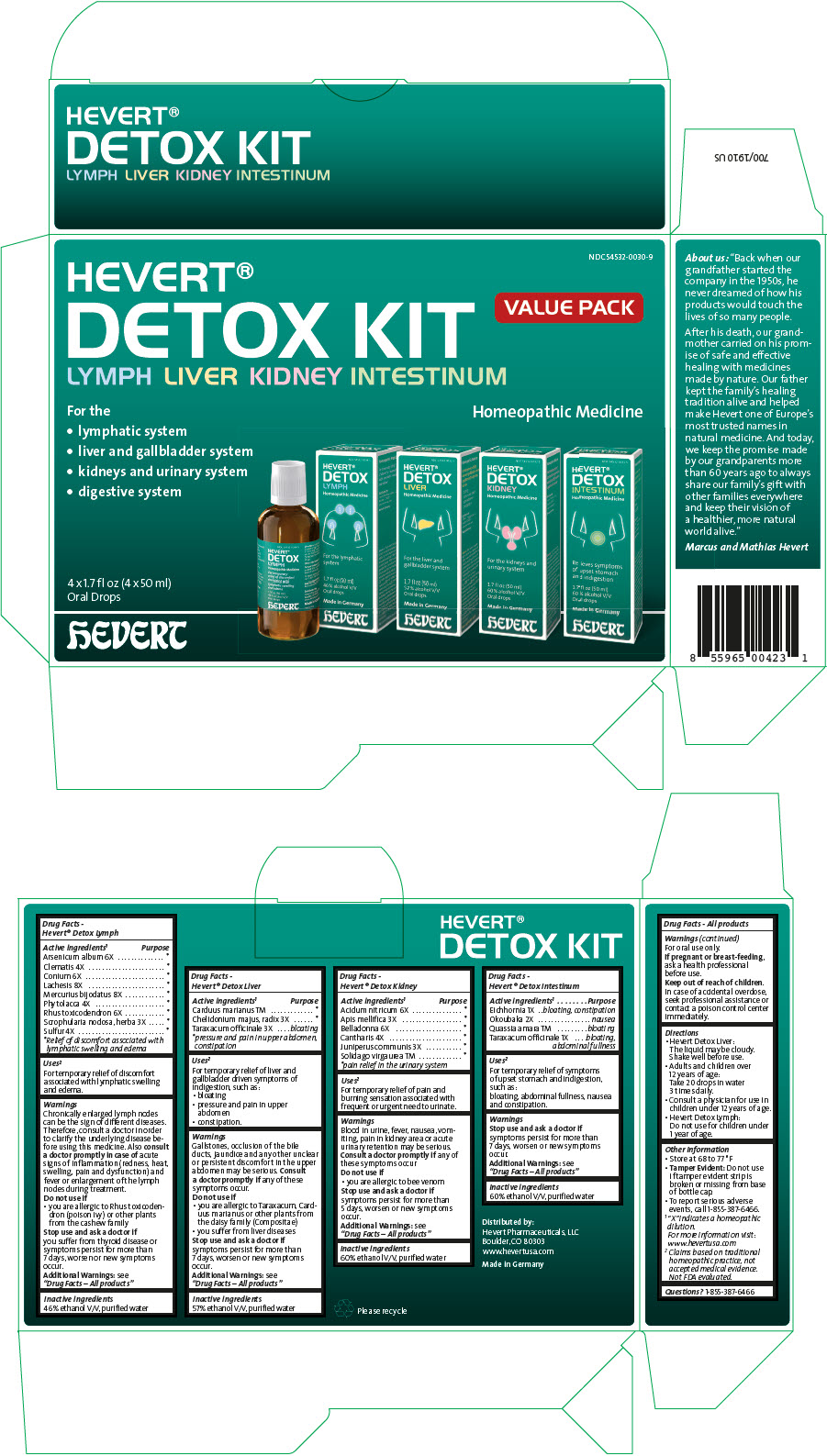
PRINCIPAL DISPLAY PANEL - Kit Carton
NDC: 54532-0030-9
HEVERT®
DETOX KIT
LYMPH LIVER KIDNEY INTESTINUMVALUE PACK
For the
- lymphatic system
- liver and gallbladder system
- kidneys and urinary system
- digestive system
Homeopathic Medicine
4 x 1.7 fl oz (4 x 50 ml)
Oral DropshEVERT
-
INGREDIENTS AND APPEARANCE
HEVERT DETOX
arsenic trioxide, clematis recta flowering top, conium maculatum flowering top, lachesis muta venom, mercuric iodide, phytolacca americana root, toxicodendron pubescens shoot, scrophularia nodosa leaf with stem, sulfur, milk thistle, chelidonium majus root, taraxacum officinale, nitric acid, apis mellifera, atropa belladonna, lytta vesicatoria, juniper berry, solidago virgaurea flowering top, eichhornia crassipes whole, okoubaka aubrevillei bark, quassia amara wood, and taraxacum officinale kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 54532-0030 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54532-0030-9 1 in 1 CARTON 09/15/2016 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 50 mL Part 2 1 BOTTLE 50 mL Part 3 1 BOTTLE 50 mL Part 4 1 BOTTLE 50 mL Part 1 of 4 HEVERT DETOX LYMPH
arsenic trioxide, clematis recta flowering top, conium maculatum flowering top, lachesis muta venom, mercuric iodide, phytolacca americana root, toxicodendron pubescens shoot, scrophularia nodosa leaf with stem, and sulfur liquidProduct Information Item Code (Source) NDC: 54532-0034 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Arsenic Trioxide (UNII: S7V92P67HO) (Arsenic Cation (3+) - UNII:C96613F5AV) Arsenic Trioxide 6 [hp_X] in 50 mL Clematis Recta Flowering top (UNII: 396421SP9F) (Clematis Recta Flowering top - UNII:396421SP9F) Clematis Recta Flowering top 4 [hp_X] in 50 mL Conium Maculatum Flowering Top (UNII: Q28R5GF371) (Conium Maculatum Flowering Top - UNII:Q28R5GF371) Conium Maculatum Flowering Top 6 [hp_X] in 50 mL Lachesis Muta Venom (UNII: VSW71SS07I) (Lachesis Muta Venom - UNII:VSW71SS07I) Lachesis Muta Venom 8 [hp_X] in 50 mL Mercuric Iodide (UNII: R03O05RB0P) (Mercuric Iodide - UNII:R03O05RB0P) Mercuric Iodide 8 [hp_X] in 50 mL Phytolacca Americana Root (UNII: 11E6VI8VEG) (Phytolacca Americana Root - UNII:11E6VI8VEG) Phytolacca Americana Root 4 [hp_X] in 50 mL Toxicodendron Pubescens shoot (UNII: 46PYZ1F82M) (Toxicodendron Pubescens shoot - UNII:46PYZ1F82M) Toxicodendron Pubescens shoot 6 [hp_X] in 50 mL Scrophularia Nodosa leaf with stem (UNII: K93UPA2CNQ) (Scrophularia Nodosa leaf with stem - UNII:K93UPA2CNQ) Scrophularia Nodosa leaf with stem 3 [hp_X] in 50 mL Sulfur (UNII: 70FD1KFU70) (Sulfur - UNII:70FD1KFU70) Sulfur 4 [hp_X] in 50 mL Inactive Ingredients Ingredient Name Strength Alcohol (UNII: 3K9958V90M) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54532-0034-5 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 06/15/2016 Part 2 of 4 HEVERT DETOX LIVER
milk thistle, chelidonium majus root, and taraxacum officinale liquidProduct Information Item Code (Source) NDC: 54532-0033 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Milk Thistle (UNII: U946SH95EE) (Milk Thistle - UNII:U946SH95EE) Milk Thistle 1 [hp_X] in 50 mL Chelidonium majus root (UNII: FLT36UCF0N) (Chelidonium majus root - UNII:FLT36UCF0N) Chelidonium majus root 3 [hp_X] in 50 mL Taraxacum officinale (UNII: 39981FM375) (Taraxacum officinale - UNII:39981FM375) Taraxacum officinale 3 [hp_X] in 50 mL Inactive Ingredients Ingredient Name Strength Alcohol (UNII: 3K9958V90M) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54532-0033-5 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 06/15/2016 Part 3 of 4 HEVERT DETOX KIDNEY
nitric acid, apis mellifera, atropa belladonna, lytta vesicatoria, juniper berry, and solidago virgaurea flowering top liquidProduct Information Item Code (Source) NDC: 54532-0032 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Nitric Acid (UNII: 411VRN1TV4) (Nitric Acid - UNII:411VRN1TV4) Nitric Acid 6 [hp_X] in 50 mL Apis Mellifera (UNII: 7S82P3R43Z) (Apis Mellifera - UNII:7S82P3R43Z) Apis Mellifera 3 [hp_X] in 50 mL Atropa Belladonna (UNII: WQZ3G9PF0H) (Atropa Belladonna - UNII:WQZ3G9PF0H) Atropa Belladonna 6 [hp_X] in 50 mL Lytta Vesicatoria (UNII: 3Q034RO3BT) (Lytta Vesicatoria - UNII:3Q034RO3BT) Lytta Vesicatoria 4 [hp_X] in 50 mL Juniper Berry (UNII: O84B5194RL) (Juniper Berry - UNII:O84B5194RL) Juniper Berry 3 [hp_X] in 50 mL Solidago Virgaurea Flowering Top (UNII: 5405K23S50) (Solidago Virgaurea Flowering Top - UNII:5405K23S50) Solidago Virgaurea Flowering Top 1 [hp_X] in 50 mL Inactive Ingredients Ingredient Name Strength Alcohol (UNII: 3K9958V90M) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54532-0032-5 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 06/15/2016 Part 4 of 4 HEVERT DETOX INTESTINUM
eichhornia crassipes whole, okoubaka aubrevillei bark, quassia amara wood, and taraxacum officinale liquidProduct Information Item Code (Source) NDC: 54532-0031 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Eichhornia Crassipes Whole (UNII: 216V7S21BH) (Eichhornia Crassipes Whole - UNII:216V7S21BH) Eichhornia Crassipes Whole 1 [hp_X] in 50 mL Okoubaka aubrevillei bark (UNII: MK2074187Z) (Okoubaka aubrevillei bark - UNII:MK2074187Z) Okoubaka aubrevillei bark 2 [hp_X] in 50 mL QUASSIA AMARA WOOD (UNII: S5249Q85HW) (QUASSIA AMARA WOOD - UNII:S5249Q85HW) QUASSIA AMARA WOOD 1 [hp_X] in 50 mL TARAXACUM OFFICINALE (UNII: 39981FM375) (TARAXACUM OFFICINALE - UNII:39981FM375) TARAXACUM OFFICINALE 1 [hp_X] in 50 mL Inactive Ingredients Ingredient Name Strength Alcohol (UNII: 3K9958V90M) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54532-0031-5 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 06/15/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved homeopathic 09/15/2016 Labeler - Hevert Pharmaceuticals LLC (078647622) Registrant - Hevert Arzneimittel GmbH & Co. KG (318100617) Establishment Name Address ID/FEI Business Operations Hevert Arzneimittel GmbH & Co. KG 318100617 MANUFACTURE(54532-0030)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.