NOT APPLICABLE- protexa cream
Not Applicable by
Drug Labeling and Warnings
Not Applicable by is a Prescription medication manufactured, distributed, or labeled by Sterling-Knight Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION:
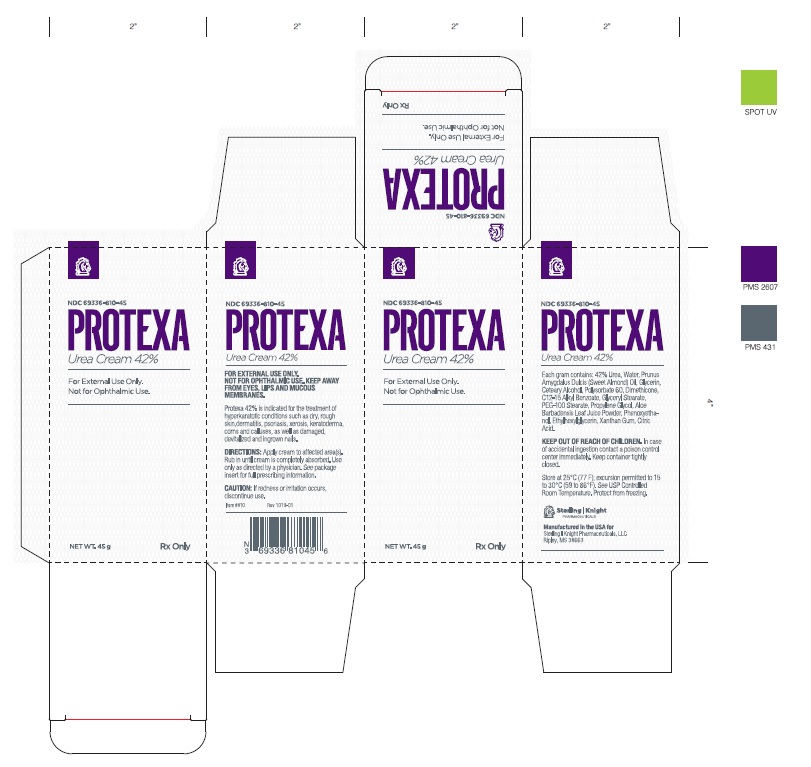
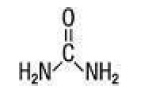
Protexa Cream (42% Urea) is a keratolytic emollient which is gentle, yet potent, tissue softener for nails and/or skin. Each gram contains 42% Urea, Water, Prunus Amygdalus Dulcis (Sweet Almond) Oil, Glycerin, Ceteary Alcohol, Polysorbate 60, Dimethicone, C12-15 Alkyl Benzoate, Glyceryl Stearate, PEG-100 Stearate, Propylene Glycol, Aloe Barbadensis Leaf Juice Powder, Phenoxyethanol, Ethylhexylglycerin, Xanthan Gum, Citric Acid. Urea Urea is a diamide of carbonic acid with the following chemical structure:
- CLINICAL PHARMACOLOGY:
- INDICATIONS AND USAGE:
- CONTRAINDICATIONS:
-
WARNINGS:
KEEP OUT OF REACH OF CHILDREN.
PRECAUTIONS: FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE.
General:
This product is to be used as directed by a physician and should not be used to treat any condition other than that for which it was prescribed. If redness or irritation occurs, discontinue use and consult a physician. -
Information for Patients:
Patients should discontinue the use of this product if the condition becomes worse or if a rash develops in the area being treated or elsewhere. Avoid contact with eyes, lips and mucous membranes.
Carcinogenesis, Mutagenesis and Impairment of Fertility: Long-term animal studies for carcinogenic potential have not been performed on this product to date. Studies on reproduction and fertility also have not been performed. -
Pregnancy:
Category C.
Animal reproduction studies have not been conducted with this product. It is also not known whether this product can affect reproduction capacity or cause fetal harm when administered to a pregnant woman. This product should be used by a pregnant woman only if clearly needed or when potential benefits outweigh potential hazards to the fetus. - Nursing Mothers:
- ADVERSE REACTIONS:
- DOSAGE AND ADMINISTRATION:
-
HOW SUPPLIED:
Protexa Cream (42% Urea) is available as follows: 45g container, NDC: 69336-810-45
KEEP THIS AND ALL MEDICATIONS OUT OF REACH OF CHILDREN.
All prescriptions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product. This product may be administered only under a physician’s supervision. There are no implied or explicit claims on the therapeutic equivalence.
Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C to 30°C (between 59°F to 86°F). Brief exposure to temperatures up to 40°C (104°F) may be tolerated provided the mean kinetic temperature does not exceed 25°C (77°F); however, such exposure should be minimized. Protect from freezing and excessive heat. Keep bottle tightly closed.
Distributed By:
Sterling-Knight Pharmaceuticals, LLC
Ripley, MS 38663Item: 810 Rev. 09/19
- Protexa Urea 42% Cream - Label
- Protexa Urea 42% Cream - Carton
-
INGREDIENTS AND APPEARANCE
NOT APPLICABLE
protexa creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69336-810 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Urea (UNII: 8W8T17847W) (UREA - UNII:8W8T17847W) Urea 42 mg in 1 g Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) ALMOND OIL (UNII: 66YXD4DKO9) GLYCERIN (UNII: PDC6A3C0OX) Polysorbate 60 (UNII: CAL22UVI4M) Dimethicone (UNII: 92RU3N3Y1O) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) PEG-100 Stearate (UNII: YD01N1999R) Propylene Glycol (UNII: 6DC9Q167V3) Aloe (UNII: V5VD430YW9) Phenoxyethanol (UNII: HIE492ZZ3T) Ethylhexylglycerin (UNII: 147D247K3P) Xanthan Gum (UNII: TTV12P4NEE) CITRIC ACID ACETATE (UNII: DSO12WL7AU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69336-810-45 1 in 1 CARTON 02/18/2020 1 45 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/18/2020 Labeler - Sterling-Knight Pharmaceuticals, LLC (079556942) Establishment Name Address ID/FEI Business Operations Sterling-Knight Pharmaceuticals, LLC 079556942 manufacture(69336-810) , label(69336-810)
Trademark Results [Not Applicable]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NOT APPLICABLE 98469202 not registered Live/Pending |
Veterans Plus Home Loans LLC 2024-03-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.