NUVIGIL- armodafinil tablet
NUVIGIL by
Drug Labeling and Warnings
NUVIGIL by is a Prescription medication manufactured, distributed, or labeled by STAT Rx USA LLC, PSS World Medical Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
NUVIGIL® (armodafinil) is a wakefulness-promoting agent for oral administration. Armodafinil is the R-enantiomer of modafinil which is a mixture of the R- and S-enantiomers. The chemical name for armodafinil is 2-[(R)-(diphenylmethyl)sulfinyl]acetamide. The molecular formula is C15H15NO2S and the molecular weight is 273.35.
The chemical structure is:
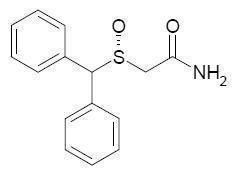
Armodafinil exists in multiple crystalline forms. Form I, which is used in NUVIGIL, is the least soluble form of armodafinil and is a white to off-white, crystalline powder that is very slightly soluble in water, sparingly soluble in acetone and soluble in methanol. At least 90% of the armodafinil particles used in NUVIGIL have a diameter less than 200 microns.
NUVIGIL tablets contain 50, 150, or 250 mg of armodafinil and the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, and pregelatinized starch.
-
CLINICAL PHARMACOLOGY
Mechanism of Action and Pharmacology
The precise mechanism(s) through which armodafinil (R-enantiomer) or modafinil (mixture of R- and S-enantiomers) promote wakefulness is unknown. Both armodafinil and modafinil have shown similar pharmacological properties in nonclinical animal and in vitro studies, to the extent tested.
At pharmacologically relevant concentrations, armodafinil does not bind to or inhibit several receptors and enzymes potentially relevant for sleep/wake regulation, including those for serotonin, dopamine, adenosine, galanin, melatonin, melanocortin, orexin-1, orphanin, PACAP or benzodiazepines, or transporters for GABA, serotonin, norepinephrine, and choline or phosphodiesterase VI, COMT, GABA transaminase, and tyrosine hydroxylase. Modafinil does not inhibit the activity of MAO-B or phosphodiesterases II-IV.
Modafinil-induced wakefulness can be attenuated by the α1-adrenergic receptor antagonist, prazosin; however, modafinil is inactive in other in vitro assay systems known to be responsive to α-adrenergic agonists such as the rat vas deferens preparation.
Armodafinil is not a direct- or indirect-acting dopamine receptor agonist. However, in vitro, both armodafinil and modafinil bind to the dopamine transporter and inhibit dopamine reuptake. For modafinil, this activity has been associated in vivo with increased extracellular dopamine levels in some brain regions of animals. In genetically engineered mice lacking the dopamine transporter (DAT), modafinil lacked wake-promoting activity, suggesting that this activity was DAT-dependent. However, the wake-promoting effects of modafinil, unlike those of amphetamine, were not antagonized by the dopamine receptor antagonist haloperidol in rats. In addition, alpha-methyl-p-tyrosine, a dopamine synthesis inhibitor, blocks the action of amphetamine, but does not block locomotor activity induced by modafinil.
Armodafinil and modafinil have wake-promoting actions similar to sympathomimetic agents including amphetamine and methylphenidate, although their pharmacologic profile is not identical to that of the sympathomimetic amines. In addition to its wake-promoting effects and ability to increase locomotor activity in animals, modafinil produces psychoactive and euphoric effects, alterations in mood, perception, thinking, and feelings typical of other CNS stimulants in humans. Modafinil has reinforcing properties, as evidenced by its self-administration in monkeys previously trained to self-administer cocaine; modafinil was also partially discriminated as stimulant-like.
Based on nonclinical studies, two major metabolites, acid and sulfone, of modafinil or armodafinil, do not appear to contribute to the CNS-activating properties of the parent compounds.
Pharmacokinetics
The active component of NUVIGIL is armodafinil, which is the longer-lived enantiomer of modafinil. NUVIGIL exhibits linear time-independent kinetics following single and multiple oral dose administration. Increase in systemic exposure is proportional over the dose range of 50 to 400 mg. No time-dependent change in kinetics was observed through 12 weeks of dosing. Apparent steady state for NUVIGIL was reached within 7 days of dosing. At steady state, the systemic exposure for NUVIGIL is 1.8 times the exposure observed after a single dose. The concentration-time profiles of the pure R-enantiomer following administration of 50 mg NUVIGIL or 100 mg PROVIGIL® (modafinil) are nearly superimposable.
Absorption
NUVIGIL is readily absorbed after oral administration. The absolute oral bioavailability was not determined due to the aqueous insolubility of armodafinil, which precluded intravenous administration. Peak plasma concentrations are attained at approximately 2 hours in the fasted state. Food effect on the overall bioavailability of NUVIGIL is considered minimal; however, time to reach peak concentration (tmax) may be delayed by approximately 2-4 hours in the fed state. Since the delay in tmax is also associated with elevated plasma levels later in time, food can potentially affect the onset and time course of pharmacologic action for NUVIGIL.
Distribution
NUVIGIL has an apparent volume of distribution of approximately 42 L. Data specific to armodafinil protein binding are not available. However, modafinil is moderately bound to plasma protein (approximately 60%), mainly to albumin. The potential for interactions of NUVIGIL with highly protein-bound drugs is considered to be minimal.
Metabolism
In vitro and in vivo data show that armodafinil undergoes hydrolytic deamidation, S-oxidation, and aromatic ring hydroxylation, with subsequent glucuronide conjugation of the hydroxylated products. Amide hydrolysis is the single most prominent metabolic pathway, with sulfone formation by cytochrome P450 (CYP) 3A4/5 being next in importance. The other oxidative products are formed too slowly in vitro to enable identification of the enzyme(s) responsible. Only two metabolites reach appreciable concentrations in plasma (i.e., R-modafinil acid and modafinil sulfone).
Data specific to NUVIGIL disposition are not available. However, modafinil is mainly eliminated via metabolism, predominantly in the liver, with less than 10% of the parent compound excreted in the urine. A total of 81% of the administered radioactivity was recovered in 11 days post-dose, predominantly in the urine (80% vs. 1.0% in the feces).
Elimination
After oral administration of NUVIGIL, armodafinil exhibits an apparent monoexponential decline from the peak plasma concentration. The apparent terminal t½ is approximately 15 hours. The oral clearance of NUVIGIL is approximately 33 mL/min.
Drug-Drug Interactions
The existence of multiple pathways for armodafinil metabolism, as well as the fact that a non-CYP-related pathway is the most rapid in metabolizing armodafinil, suggest that there is a low probability of substantive effects on the overall pharmacokinetic profile of NUVIGIL due to CYP inhibition by concomitant medications.
In vitro data demonstrated that armodafinil shows a weak inductive response for CYP1A2 and possibly CYP3A activities in a concentration-related manner and that CYP2C19 activity is reversibly inhibited by armodafinil. Other CYP activities did not appear to be affected by armodafinil. An in vitro study demonstrated that armodafinil is a substrate of P-glycoprotein.
Chronic administration of NUVIGIL at 250 mg reduced the systemic exposure to midazolam by 32% and 17% after single oral (5 mg) and intravenous (2 mg) doses, respectively, suggesting that administration of NUVIGIL moderately induces CYP3A activity. Drugs that are substrates for CYP3A4/5, such as cyclosporine, may require dosage adjustment. (SeePRECAUTIONS, Drug Interactions).
Chronic administration of NUVIGIL at 250 mg did not affect the pharmacokinetics of caffeine (200 mg), a probe substrate for CYP1A2 activity.
Coadministration of a single 400-mg dose of NUVIGIL with omeprazole (40 mg) increased systemic exposure to omeprazole by approximately 40%, indicating that armodafinil moderately inhibits CYP2C19 activity. Drugs that are substrates for CYP2C19 may require dosage reduction. (SeePRECAUTIONS, Drug Interactions).
Gender Effect
Population pharmacokinetic analysis suggests no gender effect on the pharmacokinetics of armodafinil.
Special Populations
Data specific to armodafinil in special populations are not available.
Age Effect:
A slight decrease (~20%) in the oral clearance (CL/F) of modafinil was observed in a single dose study at 200 mg in 12 subjects with a mean age of 63 years (range 53 – 72 years), but the change was considered not likely to be clinically significant. In a multiple dose study (300 mg/day) in 12 patients with a mean age of 82 years (range 67 – 87 years), the mean levels of modafinil in plasma were approximately two times those historically obtained in matched younger subjects. Due to potential effects from the multiple concomitant medications with which most of the patients were being treated, the apparent difference in modafinil pharmacokinetics may not be attributable solely to the effects of aging. However, the results suggest that the clearance of modafinil may be reduced in the elderly (SeeDOSAGE AND ADMINISTRATION).
Renal Impairment:
In a single dose 200 mg modafinil study, severe chronic renal failure (creatinine clearance ≤20 mL/min) did not significantly influence the pharmacokinetics of modafinil, but exposure to modafinil acid was increased 9-fold (SeePRECAUTIONS).
Hepatic Impairment:
The pharmacokinetics and metabolism of modafinil were examined in patients with cirrhosis of the liver (6 men and 3 women). Three patients had stage B or B+ cirrhosis and 6 patients had stage C or C+ cirrhosis (per the Child-Pugh score criteria). Clinically 8 of 9 patients were icteric and all had ascites. In these patients, the oral clearance of modafinil was decreased by about 60% and the steady state concentration was doubled compared to normal patients. The dose of NUVIGIL should be reduced in patients with severe hepatic impairment (SeePRECAUTIONS andDOSAGE AND ADMINISTRATION).
-
CLINICAL TRIALS
The effectiveness of NUVIGIL in improving wakefulness has been established in the following sleep disorders: obstructive sleep apnea (OSA), narcolepsy and shift work disorder (SWD).
For each clinical trial, a p-value of ≤ 0.05 was required for statistical significance.
Obstructive Sleep Apnea Syndrome (OSA)
The effectiveness of NUVIGIL in improving wakefulness in patients with excessive sleepiness associated with OSA was established in two 12-week, multi-center, placebo-controlled, parallel-group, double-blind studies of outpatients who met the International Classification of Sleep Disorders (ICSD) criteria for OSA (which are also consistent with the American Psychiatric Association DSM-IV criteria). These criteria include either, 1) excessive sleepiness or insomnia, plus frequent episodes of impaired breathing during sleep, and associated features such as loud snoring, morning headaches or dry mouth upon awakening; or 2) excessive sleepiness or insomnia; and polysomnography demonstrating one of the following: more than five obstructive apneas, each greater than 10 seconds in duration, per hour of sleep; and one or more of the following: frequent arousals from sleep associated with the apneas, bradytachycardia, or arterial oxygen desaturation in association with the apneas. In addition, for entry into these studies, all patients were required to have excessive sleepiness as demonstrated by a score ≥ 10 on the Epworth Sleepiness Scale, despite treatment with continuous positive airway pressure (CPAP). Evidence that CPAP was effective in reducing episodes of apnea/hypopnea was required along with documentation of CPAP use.
Patients were required to be compliant with CPAP, defined as CPAP use ≥ 4 hours/night on ≥ 70% of nights. CPAP use continued throughout the study. In both studies, the primary measures of effectiveness were 1) sleep latency, as assessed by the Maintenance of Wakefulness Test (MWT) and 2) the change in the patient’s overall disease status, as measured by the Clinical Global Impression of Change (CGI-C) at the final visit. For a successful trial both measures had to show statistically significant improvement.
The MWT measures latency (in minutes) to sleep onset. An extended MWT was performed with test sessions at 2 hour intervals between 9AM and 7PM. The primary analysis was the average of the sleep latencies from the first four test sessions (9AM to 3PM). For each test session, the subject was asked to attempt to remain awake without using extraordinary measures. Each test session was terminated after 30 minutes if no sleep occurred or immediately after sleep onset. The CGI-C is a 7-point scale, centered at No Change, and ranging from Very Much Worse to Very Much Improved. Evaluators were not given any specific guidance about the criteria they were to apply when rating patients.
In the first study, a total of 395 patients with OSA were randomized to receive NUVIGIL 150 mg/day, NUVIGIL 250 mg/day or matching placebo. Patients treated with NUVIGIL showed a statistically significant improvement in the ability to remain awake compared to placebo-treated patients as measured by the MWT at final visit. A statistically significant greater number of patients treated with NUVIGIL showed improvement in overall clinical condition as rated by the CGI-C scale at final visit. The average sleep latencies (in minutes) in the MWT at baseline for the trials are shown in Table 1 below, along with the average change from baseline on the MWT at final visit. The percentages of patients who showed any degree of improvement on the CGI-C in the clinical trials are shown in Table 2 below. The two doses of NUVIGIL produced statistically significant effects of similar magnitudes on the MWT, and also on the CGI-C.
In the second study, 263 patients with OSA were randomized to either NUVIGIL 150 mg/day or placebo. Patients treated with NUVIGIL showed a statistically significant improvement in the ability to remain awake compared to placebo-treated patients as measured by the MWT [Table 1]. A statistically significant greater number of patients treated with NUVIGIL showed improvement in overall clinical condition as rated by the CGI-C scale [Table 2].
Nighttime sleep measured with polysomnography was not affected by the use of NUVIGIL in either study.
Narcolepsy
The effectiveness of NUVIGIL in improving wakefulness in patients with excessive sleepiness (ES) associated with narcolepsy was established in one 12-week, multi-center, placebo-controlled, parallel-group, double-blind study of outpatients who met the ICSD criteria for narcolepsy. A total of 196 patients were randomized to receive NUVIGIL 150 or 250 mg/day, or matching placebo. The ICSD criteria for narcolepsy include either 1) recurrent daytime naps or lapses into sleep that occur almost daily for at least three months, plus sudden bilateral loss of postural muscle tone in association with intense emotion (cataplexy), or 2) a complaint of excessive sleepiness or sudden muscle weakness with associated features: sleep paralysis, hypnagogic hallucinations, automatic behaviors, disrupted major sleep episode; and polysomnography demonstrating one of the following: sleep latency less than 10 minutes or rapid eye movement (REM) sleep latency less than 20 minutes and a Multiple Sleep Latency Test (MSLT) that demonstrates a mean sleep latency of less than 5 minutes and two or more sleep onset REM periods and no medical or mental disorder accounts for the symptoms. For entry into these studies, all patients were required to have objectively documented excessive daytime sleepiness, via MSLT with a sleep latency of 6 minutes or less and the absence of any other clinically significant active medical or psychiatric disorder. The MSLT, an objective polysomnographic assessment of the patient’s ability to fall asleep in an unstimulating environment, measured latency (in minutes) to sleep onset averaged over 4 test sessions at 2-hour intervals. For each test session, the subject was told to lie quietly and attempt to sleep. Each test session was terminated after 20 minutes if no sleep occurred or immediately after sleep onset.
The primary measures of effectiveness were: 1) sleep latency as assessed by the Maintenance of Wakefulness Test (MWT) and 2) the change in the patient’s overall disease status, as measured by the Clinical Global Impression of Change (CGI-C) at the final visit (See CLINICAL TRIALS, OSA section above for a description of these measures). Each MWT test session was terminated after 20 minutes if no sleep occurred or immediately after sleep onset in this study.
Patients treated with NUVIGIL showed a statistically significantly enhanced ability to remain awake on the MWT at each dose compared to placebo at final visit [Table 1]. A statistically significant greater number of patients treated with NUVIGIL at each dose showed improvement in overall clinical condition as rated by the CGI-C scale at final visit [Table 2].
The two doses of NUVIGIL produced statistically significant effects of similar magnitudes on the CGI-C. Although a statistically significant effect on the MWT was observed for each dose, the magnitude of effect was observed to be greater for the higher dose.
Nighttime sleep measured with polysomnography was not affected by the use of NUVIGIL.
Shift Work Disorder (SWD)
The effectiveness of NUVIGIL in improving wakefulness in patients with excessive sleepiness associated with SWD was demonstrated in a 12-week, multi-center, double-blind, placebo-controlled, parallel-group, clinical trial. A total of 254 patients with chronic SWD were randomized to receive NUVIGIL 150 mg/day or placebo. All patients met the ICSD criteria for chronic SWD [which are consistent with the American Psychiatric Association DSM-IV criteria for Circadian Rhythm Sleep Disorder: Shift Work Type]. These criteria include 1) either: a) a primary complaint of excessive sleepiness or insomnia which is temporally associated with a work period (usually night work) that occurs during the habitual sleep phase, or b) polysomnography and the MSLT demonstrate loss of a normal sleep-wake pattern (i.e., disturbed chronobiological rhythmicity); and 2) no other medical or mental disorder accounts for the symptoms, and 3) the symptoms do not meet criteria for any other sleep disorder producing insomnia or excessive sleepiness (e.g., time zone change [jet lag] syndrome).
It should be noted that not all patients with a complaint of sleepiness who are also engaged in shift work meet the criteria for the diagnosis of SWD. In the clinical trial, only patients who were symptomatic for at least 3 months were enrolled.
Enrolled patients were also required to work a minimum of 5 night shifts per month, have excessive sleepiness at the time of their night shifts (MSLT score ≤ 6 minutes), and have daytime insomnia documented by a daytime polysomnogram (PSG).
The primary measures of effectiveness were 1) sleep latency, as assessed by the Multiple Sleep Latency Test (MSLT) performed during a simulated night shift at the final visit, and 2) the change in the patient’s overall disease status, as measured by the Clinical Global Impression of Change (CGI-C) at the final visit. (SeeCLINICAL TRIALS, Narcolepsy andOSA sections above for description of these measures).
Patients treated with NUVIGIL showed a statistically significant prolongation in the time to sleep onset compared to placebo-treated patients, as measured by the nighttime MSLT at final visit [Table 1]. A statistically significant greater number of patients treated with NUVIGIL showed improvement in overall clinical condition as rated by the CGI-C scale at final visit [Table 2].
Daytime sleep measured with polysomnography was not affected by the use of NUVIGIL.
Table 1. Average Baseline Sleep Latency and Change from Baseline at Final Visit (MWT and MSLT in minutes) Disorder Measure NUVIGIL
150 mg*NUVIGIL
250 mg*Placebo *Significantly different than placebo for all trials (p<0.05)
Baseline Change
from BaselineBaseline Change
from BaselineBaseline Change from
BaselineOSA I MWT 21.5 1.7 23.3 2.2 23.2 -1.7 OSA II MWT 23.7 2.3 - - 23.3 -1.3 Narcolepsy MWT 12.1 1.3 9.5 2.6 12.5 -1.9 SWD MSLT 2.3 3.1 - - 2.4 0.4 Table 2. Clinical Global Impression of Change (CGI-C) (Percent of Patients Who Improved at Final Visit) Disorder NUVIGIL
150 mg*NUVIGIL
250 mg*Placebo
*Significantly different than placebo for all trials (p<0.05)
OSA I 71% 74% 37% OSA II 71% - 53% Narcolepsy 69% 73% 33% SWD 79% - 59% -
INDICATIONS AND USAGE
NUVIGIL is indicated to improve wakefulness in patients with excessive sleepiness associated with obstructive sleep apnea, narcolepsy and shift work disorder.
In OSA, NUVIGIL is indicated as an adjunct to standard treatment(s) for the underlying obstruction. If continuous positive airway pressure (CPAP) is the treatment of choice for a patient, a maximal effort to treat with CPAP for an adequate period of time should be made prior to initiating NUVIGIL. If NUVIGIL is used adjunctively with CPAP, the encouragement of and periodic assessment of CPAP compliance is necessary.
In all cases, careful attention to the diagnosis and treatment of the underlying sleep disorder(s) is of utmost importance. Prescribers should be aware that some patients may have more than one sleep disorder contributing to their excessive sleepiness.
The effectiveness of NUVIGIL in long-term use (greater than 12 weeks) has not been systematically evaluated in placebo-controlled trials. The physician who elects to prescribe NUVIGIL for an extended time in patients should periodically re-evaluate long-term usefulness for the individual patient.
- CONTRAINDICATIONS
-
WARNINGS
Serious Rash, including Stevens-Johnson Syndrome
Serious rash requiring hospitalization and discontinuation of treatment has been reported in adults in association with the use of modafinil and armodafinil and in children in association with the use of modafinil.
Armodafinil has not been studied in pediatric patients in any setting and is not approved for use in pediatric patients for any indication.
In clinical trials of modafinil (a racemic mixture of S and R enantiomers), the incidence of rash resulting in discontinuation was approximately 0.8% (13 per 1,585) in pediatric patients (age <17 years); these rashes included 1 case of possible Stevens-Johnson Syndrome (SJS) and 1 case of apparent multi-organ hypersensitivity reaction. Several of the cases were associated with fever and other abnormalities (e.g., vomiting, leukopenia). The median time to rash that resulted in discontinuation was 13 days. No such cases were observed among 380 pediatric patients who received placebo. No serious skin rashes have been reported in adult clinical trials (0 per 4,264) of modafinil. Rare cases of serious or life-threatening rash, including SJS, Toxic Epidermal Necrolysis (TEN), and Drug Rash with Eosinophilia and Systemic Symptoms (DRESS) have been reported in adults and children in worldwide post-marketing experience with modafinil. The reporting rate of TEN and SJS associated with modafinil use, which is generally accepted to be an underestimate due to underreporting, exceeds the background incidence rate. Estimates of the background incidence rate for these serious skin reactions in the general population range between 1 to 2 cases per million-person years.
No serious skin rashes have been reported in adult clinical trials (0 per 1,595) of armodafinil. However, cases of serious rash similar to those observed with modafinil including skin and mouth blistering have been reported in adults in postmarketing experience.
There are no factors that are known to predict the risk of occurrence or the severity of rash associated with armodafinil or modafinil. Nearly all cases of serious rash associated with these drugs occurred within 1 to 5 weeks after treatment initiation. However, isolated cases have been reported after prolonged treatment with modafinil (e.g., 3 months). Accordingly, duration of therapy cannot be relied upon as a means to predict the potential risk heralded by the first appearance of a rash.
Although benign rashes also occur with armodafinil, it is not possible to reliably predict which rashes will prove to be serious. Accordingly, armodafinil should ordinarily be discontinued at the first sign of rash, unless the rash is clearly not drug-related. Discontinuation of treatment may not prevent a rash from becoming life-threatening or permanently disabling or disfiguring.
Angioedema and Anaphylactoid Reactions
One serious case of angioedema and one case of hypersensitivity (with rash, dysphagia, and bronchospasm), were observed among 1,595 patients treated with armodafinil. Patients should be advised to discontinue therapy and immediately report to their physician any signs or symptoms suggesting angioedema or anaphylaxis (e.g., swelling of face, eyes, lips, tongue or larynx; difficulty in swallowing or breathing; hoarseness).
Multi-organ Hypersensitivity Reactions
Multi-organ hypersensitivity reactions, including at least one fatality in postmarketing experience, have occurred in close temporal association (median time to detection 13 days: range 4-33) to the initiation of modafinil. A similar risk of multi-organ hypersensitivity reactions with armodafinil cannot be ruled out.
Although there have been a limited number of reports, multi-organ hypersensitivity reactions may result in hospitalization or be life-threatening. There are no factors that are known to predict the risk of occurrence or the severity of multi-organ hypersensitivity reactions associated with modafinil. Signs and symptoms of this disorder were diverse; however, patients typically, although not exclusively, presented with fever and rash associated with other organ system involvement. Other associated manifestations included myocarditis, hepatitis, liver function test abnormalities, hematological abnormalities (e.g., eosinophilia, leukopenia, thrombocytopenia), pruritus, and asthenia. Because multi-organ hypersensitivity is variable in its expression, other organ system symptoms and signs, not noted here, may occur.
If a multi-organ hypersensitivity reaction is suspected, NUVIGIL should be discontinued. Although there are no case reports to indicate cross-sensitivity with other drugs that produce this syndrome, the experience with drugs associated with multi-organ hypersensitivity would indicate this to be a possibility.
Persistent Sleepiness
Patients with abnormal levels of sleepiness who take NUVIGIL should be advised that their level of wakefulness may not return to normal. Patients with excessive sleepiness, including those taking NUVIGIL, should be frequently reassessed for their degree of sleepiness and, if appropriate, advised to avoid driving or any other potentially dangerous activity. Prescribers should also be aware that patients may not acknowledge sleepiness or drowsiness until directly questioned about drowsiness or sleepiness during specific activities.
Psychiatric Symptoms
Psychiatric adverse experiences have been reported in patients treated with modafinil. Modafinil and armodafinil (NUVIGIL) are very closely related. Therefore, the incidence and type of psychiatric symptoms associated with armodafinil are expected to be similar to the incidence and type of these events with modafinil.
Postmarketing adverse events associated with the use of modafinil have included mania, delusions, hallucinations, suicidal ideation and aggression, some resulting in hospitalization. Many, but not all, patients had a prior psychiatric history. One healthy male volunteer developed ideas of reference, paranoid delusions, and auditory hallucinations in association with multiple daily 600 mg doses of modafinil and sleep deprivation. There was no evidence of psychosis 36 hours after drug discontinuation.
In the controlled trial NUVIGIL database, anxiety, agitation, nervousness, and irritability were reasons for treatment discontinuation more often in patients on NUVIGIL compared to placebo (NUVIGIL 1.2% and placebo 0.3%). In the NUVIGIL controlled studies, depression was also a reason for treatment discontinuation more often in patients on NUVIGIL compared to placebo (NUVIGIL 0.6% and placebo 0.2%). Two cases of suicide ideation were observed in clinical trials. Caution should be exercised when NUVIGIL is given to patients with a history of psychosis, depression, or mania. If psychiatric symptoms develop in association with NUVIGIL administration, consider discontinuing NUVIGIL.
-
PRECAUTIONS
Diagnosis of Sleep Disorders
NUVIGIL should be used only in patients who have had a complete evaluation of their excessive sleepiness, and in whom a diagnosis of either narcolepsy, OSA, and/or SWD has been made in accordance with ICSD or DSM diagnostic criteria (SeeCLINICAL TRIALS). Such an evaluation usually consists of a complete history and physical examination, and it may be supplemented with testing in a laboratory setting. Some patients may have more than one sleep disorder contributing to their excessive sleepiness (e.g., OSA and SWD coincident in the same patient).
CPAP Use in Patients with OSA
In OSA, NUVIGIL is indicated as an adjunct to standard treatment(s) for the underlying obstruction. If continuous positive airway pressure (CPAP) is the treatment of choice for a patient, a maximal effort to treat with CPAP for an adequate period of time should be made prior to initiating NUVIGIL. If NUVIGIL is used adjunctively with CPAP, the encouragement of and periodic assessment of CPAP compliance is necessary. There was a slight trend for reduced CPAP use over time (mean reduction of 18 minutes for patients treated with NUVIGIL and a 6 minute reduction for placebo-treated patients from a mean baseline use of 6.9 hours per night) in NUVIGIL trials.
General
Although NUVIGIL has not been shown to produce functional impairment, any drug affecting the CNS may alter judgment, thinking or motor skills. Patients should be cautioned about operating an automobile or other hazardous machinery until they are reasonably certain that NUVIGIL therapy will not adversely affect their ability to engage in such activities.
Cardiovascular System
NUVIGIL has not been evaluated or used to any appreciable extent in patients with a recent history of myocardial infarction or unstable angina, and such patients should be treated with caution.
In clinical studies of PROVIGIL, signs and symptoms including chest pain, palpitations, dyspnea and transient ischemic T-wave changes on ECG were observed in three subjects in association with mitral valve prolapse or left ventricular hypertrophy. It is recommended that NUVIGIL tablets not be used in patients with a history of left ventricular hypertrophy or in patients with mitral valve prolapse who have experienced the mitral valve prolapse syndrome when previously receiving CNS stimulants. Signs of mitral valve prolapse syndrome include but are not limited to ischemic ECG changes, chest pain, or arrhythmia. If new onset of any of these symptoms occurs, consider cardiac evaluation.
Blood pressure monitoring in short-term (≤ 3 months) controlled trials showed only small average increases in mean systolic and diastolic blood pressure in patients receiving NUVIGIL as compared to placebo (1.2 to 4.3 mmHg in the various experimental groups). There was also a slightly greater proportion of patients on NUVIGIL requiring new or increased use of antihypertensive medications (2.9%) compared to patients on placebo (1.8%). Increased monitoring of blood pressure may be appropriate in patients on NUVIGIL.
Patients Using Steroidal Contraceptives
The effectiveness of steroidal contraceptives may be reduced when used with NUVIGIL and for one month after discontinuation of therapy (SeePRECAUTIONS, Drug Interactions). Alternative or concomitant methods of contraception are recommended for patients treated with NUVIGIL and for one month after discontinuation of NUVIGIL treatment.
Patients Using Cyclosporine
The blood levels of cyclosporine may be reduced when used with NUVIGIL (See PRECAUTIONS, Drug Interactions). Monitoring of circulating cyclosporine concentrations and appropriate dosage adjustment for cyclosporine should be considered when these drugs are used concomitantly.
Patients with Severe Hepatic Impairment
In patients with severe hepatic impairment, with or without cirrhosis (SeeCLINICAL PHARMACOLOGY), NUVIGIL should be administered at a reduced dose (SeeDOSAGE AND ADMINISTRATION).
Patients with Severe Renal Impairment
There is inadequate information to determine safety and efficacy of dosing in patients with severe renal impairment (For pharmacokinetics in renal impairment, seeCLINICAL PHARMACOLOGY.)
Elderly Patients
In elderly patients, elimination of armodafinil and its metabolites may be reduced as a consequence of aging. Therefore, consideration should be given to the use of lower doses in this population (SeeCLINICAL PHARMACOLOGYandDOSAGE AND ADMINISTRATION).
Information for Patients
Physicians are advised to discuss the following issues with patients for whom they prescribe NUVIGIL.
NUVIGIL is indicated for patients who have abnormal levels of sleepiness. NUVIGIL has been shown to improve, but not eliminate, this abnormal tendency to fall asleep. Therefore, patients should not alter their previous behavior with regard to potentially dangerous activities (e.g., driving, operating machinery) or other activities requiring appropriate levels of wakefulness, until and unless treatment with NUVIGIL has been shown to produce levels of wakefulness that permit such activities. Patients should be advised that NUVIGIL is not a replacement for sleep.
Patients should be informed that it may be critical that they continue to take their previously prescribed treatments (e.g., patients with OSA receiving CPAP should continue to do so).
Patients should be informed of the availability of a Medication Guide, and they should be instructed to read it prior to taking NUVIGIL. The complete text of the Medication Guide is provided at the end of this labeling.
Patients should be advised to contact their physician if they experience rash, depression, anxiety, or signs of psychosis or mania.
Pregnancy
Patients should be advised to notify their physician if they become pregnant or intend to become pregnant during therapy. Patients should be cautioned regarding the potential increased risk of pregnancy when using steroidal contraceptives (including depot or implantable contraceptives) with NUVIGIL and for one month after discontinuation of therapy (SeeCarcinogenesis, Mutagenesis, Impairment of Fertility andPregnancy).
Concomitant Medication
Patients should be advised to inform their physician if they are taking, or plan to take, any prescription or over-the-counter drugs, because of the potential for interactions between NUVIGIL and other drugs.
Alcohol
Patients should be advised that the use of NUVIGIL in combination with alcohol has not been studied. Patients should be advised that it is prudent to avoid alcohol while taking NUVIGIL.
Allergic Reactions
Patients should be advised to stop taking NUVIGIL and to notify their physician if they develop a rash, hives, mouth sores, blisters, peeling skin, trouble swallowing or breathing or a related allergic phenomenon.
Drug Interactions
Potential Interactions with Drugs That Inhibit, Induce, or Are Metabolized by Cytochrome P450 Isoenzymes and Other Hepatic Enzymes
Due to the partial involvement of CYP3A enzymes in the metabolic elimination of armodafinil, coadministration of potent inducers of CYP3A4/5 (e.g., carbamazepine, phenobarbital, rifampin) or inhibitors of CYP3A4/5 (e.g., ketoconazole, erythromycin) could alter the plasma levels of armodafinil.
The Potential of NUVIGIL to Alter the Metabolism of Other Drugs by Enzyme Induction or Inhibition
Drugs Metabolized by CYP1A2
In vitro data demonstrated that armodafinil shows a weak inductive response for CYP1A2 and possibly CYP3A activities in a concentration related manner and demonstrated that CYP2C19 activity is reversibly inhibited by armodafinil. However, the effect on CYP1A2 activity was not observed clinically in an interaction study performed with caffeine (See Pharmacokinetics, Drug-Drug Interactions).
Drugs Metabolized by CYP3A4/5 (e.g., cyclosporine, ethinyl estradiol, midazolam and triazolam)
Chronic administration of NUVIGIL resulted in moderate induction of CYP3A activity. Hence, the effectiveness of drugs that are substrates for CYP3A enzymes (e.g., cyclosporine, ethinyl estradiol, midazolam and triazolam) may be reduced after initiation of concurrent treatment with NUVIGIL. A 32% reduction in systemic exposure of oral midazolam was seen upon concomitant administration of armodafinil with midazolam. Dose adjustment may be required (See Pharmacokinetics, Drug-Drug Interactions). Such effects (reduced concentrations) were also seen upon concomitant administration of modafinil with cyclosporine, ethinyl estradiol, and triazolam.
Drugs Metabolized by CYP2C19 (e.g., omeprazole, diazepam, phenytoin, and propranolol)
Administration of NUVIGIL resulted in moderate inhibition of CYP2C19 activity. Hence, dosage reduction may be required for some drugs that are substrates for CYP2C19 (e.g., phenytoin, diazepam, and propranolol, omeprazole and clomipramine) when used concurrently with NUVIGIL. A 40% increase in exposure was seen upon concomitant administration of armodafinil with omeprazole. (SeePharmacokinetics, Drug-Drug Interactions).
Interactions with CNS Active Drugs
Data specific to armodafinil drug-drug interaction potential with CNS active drugs are not available. However, the following available drug-drug interaction information on modafinil should be applicable to armodafinil (SeeDESCRIPTIONand CLINICAL PHARMACOLOGY).
Concomitant administration of modafinil with methylphenidate, or dextroamphetamine produced no significant alterations on the pharmacokinetic profile of modafinil or either stimulant, even though the absorption of modafinil was delayed for approximately one hour.
Concomitant modafinil or clomipramine did not alter the PK profile of either drug; however, one incident of increased levels of clomipramine and its active metabolite desmethylclomipramine was reported in a patient with narcolepsy during treatment with modafinil.
Data specific to armodafinil or modafinil drug-drug interaction potential with Monoamine Oxidase (MAO) inhibitors are not available. Therefore, caution should be used when concomitantly administering MAO inhibitors and NUVIGIL.
Interactions with Other Drugs
Data specific to armodafinil drug-drug interaction potential for additional other drugs are not available. However, the following available drug-drug interaction information on modafinil should be applicable to armodafinil.
Warfarin
Concomitant administration of modafinil with warfarin did not produce significant changes in the pharmacokinetic profiles of R- and S-warfarin. However, since only a single dose of warfarin was tested in this study, a pharmacodynamic interaction cannot be ruled out. Therefore, more frequent monitoring of prothrombin times/INR should be considered whenever NUVIGIL is coadministered with warfarin.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies have not been conducted with armodafinil alone. Carcinogenicity studies were conducted in which modafinil was administered in the diet to mice for 78 weeks and to rats for 104 weeks at doses of 6, 30, and 60 mg/kg/day. The highest dose studied represents 1.5 (mouse) or 3 (rat) times greater than the recommended adult human daily dose of modafinil (200 mg) on a mg/m2 basis. There was no evidence of tumorigenesis associated with modafinil administration in these studies. However, since the mouse study used an inadequate high dose that was not representative of a maximum tolerated dose, a subsequent carcinogenicity study was conducted in the Tg.AC transgenic mouse. Doses evaluated in the Tg.AC assay were 125, 250, and 500 mg/kg/day, administered dermally. There was no evidence of tumorigenicity associated with modafinil administration; however, this dermal model may not adequately assess the carcinogenic potential of an orally administered drug.
Mutagenesis
Armodafinil was evaluated in an in vitro bacterial reverse mutation assay and in an in vitro mammalian chromosomal aberration assay in human lymphocytes. Armodafinil was negative in these assays, both in the absence and presence of metabolic activation. Modafinil demonstrated no evidence of mutagenic or clastogenic potential in a series of in vitro (i.e., bacterial reverse mutation assay, mouse lymphoma tk assay, chromosomal aberration assay in human lymphocytes, cell transformation assay in BALB/3T3 mouse embryo cells) assays in the absence or presence of metabolic activation, or in vivo (mouse bone marrow micronucleus) assays. Modafinil was also negative in the unscheduled DNA synthesis assay in rat hepatocytes.
Impairment of Fertility
A fertility and early embryonic development (to implantation) study was not conducted with armodafinil alone.
Oral administration of modafinil (doses of up to 480 mg/kg/day) to male and female rats prior to and throughout mating, and continuing in females through day 7 of gestation produced an increase in the time to mate at the highest dose; no effects were observed on other fertility or reproductive parameters. The no-effect dose of 240 mg/kg/day was associated with a plasma modafinil exposure (AUC) approximately equal to that in humans at the recommended dose of 200 mg.
Pregnancy
Pregnancy Category C
In studies conducted in rats (armodafinil, modafinil) and rabbits (modafinil), developmental toxicity was observed at clinically relevant exposures.
Oral administration of armodafinil (60, 200, or 600 mg/kg/day) to pregnant rats throughout the period of organogenesis resulted in increased incidences of fetal visceral and skeletal variations at the intermediate dose or greater and decreased fetal body weights at the highest dose. The no-effect dose for rat embryofetal developmental toxicity was associated with a plasma armodafinil exposure (AUC) approximately 0.03 times the AUC in humans at the maximum recommended daily dose of 250 mg.
Modafinil (50, 100, or 200 mg/kg/day) administered orally to pregnant rats throughout the period of organogenesis caused, in the absence of maternal toxicity, an increase in resorptions and an increased incidence of visceral and skeletal variations in the offspring at the highest dose. The higher no-effect dose for rat embryofetal developmental toxicity was associated with a plasma modafinil exposure approximately 0.5 times the AUC in humans at the recommended daily dose (RHD) of 200 mg. However, in a subsequent study of up to 480 mg/kg/day (plasma modafinil exposure approximately 2 times the AUC in humans at the RHD) no adverse effects on embryofetal development were observed.
Modafinil administered orally to pregnant rabbits throughout the period of organogenesis at doses of up to 100 mg/kg/day (plasma modafinil AUC approximately equal to the AUC in humans at the RHD) had no effect on embryofetal development; however, the doses used were too low to adequately assess the effects of modafinil on embryofetal development. In a subsequent developmental toxicity study evaluating doses of 45, 90, and 180 mg/kg/day in pregnant rabbits, the incidences of fetal structural alterations and embryofetal death were increased at the highest dose. The highest no-effect dose for developmental toxicity was associated with a plasma modafinil AUC approximately equal to the AUC in humans at the RHD.
Modafinil administration to rats throughout gestation and lactation at oral doses of up to 200 mg/kg/day resulted in decreased viability in the offspring at doses greater than 20 mg/kg/day (plasma modafinil AUC approximately 0.1 times the AUC in humans at the RHD). No effects on postnatal developmental and neurobehavioral parameters were observed in surviving offspring.
There are no adequate and well-controlled studies of either armodafinil or modafinil in pregnant women. Two cases of intrauterine growth retardation and one case of spontaneous abortion have been reported in association with armodafinil and modafinil. Although the pharmacology of armodafinil is not identical to that of the sympathomimetic amines, it does share some pharmacologic properties with this class.
Certain of these drugs have been associated with intrauterine growth retardation and spontaneous abortions. Whether the cases reported with armodafinil are drug-related is unknown.
Armodafinil or modafinil should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Registry: A pregnancy registry has been established to collect information on the pregnancy outcomes of women exposed to NUVIGIL. Healthcare providers are encouraged to register pregnant patients, or pregnant women may enroll themselves in the registry by calling 1-866-404-4106 (toll free).
Labor and Delivery
The effect of armodafinil on labor and delivery in humans has not been systematically investigated.
Nursing Mothers
It is not known whether armodafinil or its metabolites are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when NUVIGIL tablets are administered to a nursing woman.
Pediatric Use
Safety and effectiveness of armodafinil use in individuals below 17 years of age have not been established. Serious rash has been seen in pediatric patients receiving modafinil (SeeWARNINGS, Serious Rash, including Stevens-Johnson Syndrome).
Geriatric Use
In elderly patients, elimination of armodafinil and its metabolites may be reduced as a consequence of aging. Therefore, consideration should be given to the use of lower doses in this population (See CLINICAL PHARMACOLOGY and PRECAUTIONS).
-
ADVERSE REACTIONS
Armodafinil has been evaluated for safety in over 1100 patients with excessive sleepiness associated with primary disorders of sleep and wakefulness. In clinical trials, NUVIGIL has been found to be generally well tolerated and most adverse experiences were mild to moderate.
In the placebo-controlled clinical studies, the most commonly observed adverse events (≥ 5%) associated with the use of NUVIGIL occurring more frequently than in the placebo-treated patients were headache, nausea, dizziness, and insomnia. The adverse event profile was similar across the studies.
In the placebo-controlled clinical trials, 44 of the 645 patients (7%) who received NUVIGIL discontinued due to an adverse experience compared to 16 of the 445 (4%) of patients that received placebo. The most frequent reason for discontinuation was headache (1%).
Incidence in Controlled Trials
The following table (Table 3) presents the adverse experiences that occurred at a rate of 1% or more and were more frequent in patients treated with NUVIGIL than in placebo group patients in the placebo-controlled clinical trials.
The prescriber should be aware that the figures provided below cannot be used to predict the frequency of adverse experiences in the course of usual medical practice, where patient characteristics and other factors may differ from those occurring during clinical studies. Similarly, the cited frequencies cannot be directly compared with figures obtained from other clinical investigations involving different treatments, uses, or investigators. Review of these frequencies, however, provides prescribers with a basis to estimate the relative contribution of drug and non-drug factors to the incidence of adverse events in the population studied.
Table 3. Incidence > 1% (In Percent) Of Treatment-Emergent Adverse Experiences In Parallel-Group, Placebo-Controlled Clinical Trialsª In OSA, Narcolepsy and SWD With NUVIGIL (150 mg and 250 mg)
System Organ Class
MedDRA preferred termNUVIGIL
(Percent, N=645)Placebo
(Percent, N=445)Cardiac Disorders Palpitations 2 1 Gastrointestinal Disorders Nausea 7 3 Diarrhea 4 2 Dry Mouth 4 1 Dyspepsia 2 0 Abdominal Pain Upper 2 1 Constipation 1 0 Vomiting 1 0 Loose Stools 1 0 General Disorders And Administration Site Conditions Fatigue 2 1 Thirst 1 0 Influenza-Like Illness 1 0 Pain 1 0 Pyrexia 1 0 Immune System Disorders Seasonal Allergy 1 0 Investigations Gamma-Glutamyltransferase Increased 1 0 Heart Rate Increased 1 0 Metabolism And Nutrition Disorders Anorexia 1 0 Decreased Appetite 1 0 Nervous System Disorders Headache 17 9 Dizziness 5 2 Disturbance In Attention 1 0 Tremor 1 0 Migraine 1 0 Paresthesia 1 0 Psychiatric Disorders Insomnia 5 1 Anxiety 4 1 Depression 2 0 Agitation 1 0 Nervousness 1 0 Depressed Mood 1 0 Renal And Urinary Disorders Polyuria 1 0 Respiratory, Thoracic And Mediastinal Disorders Dyspnea 1 0 Skin And Subcutaneous Tissue Disorders Rash 2 0 Contact Dermatitis 1 0 Hyperhydrosis 1 0 ª Four double-blind, placebo-controlled clinical studies in SWD, OSA, and narcolepsy; incidence is rounded to the nearest whole percent. Included are only those events for which NUVIGIL incidence is greater than that of placebo. Dose Dependency of Adverse Events
In the placebo-controlled clinical trials which compared doses of 150 mg/day and 250 mg/day of NUVIGIL and placebo, the only adverse events that appeared to be dose-related were headache, rash, depression, dry mouth, insomnia, and nausea.
Table 4. Incidence (In Percent) Of Dose-Dependent , Treatment-Emergent Adverse Experiences By Dose and By Treatment In Parallel-Group, Placebo-ControlledClinical Trialsa In OSA, Narcolepsy and SWD With NUVIGIL (150 mg and 250 mg)
System Organ Class
MedDRA preferred termNUVIGIL
250 mg
(Percent,
N=198)NUVIGIL
150 mg
(Percent, N=447)NUVIGIL Combined
(Percent, N=645)Placebo
(Percent,
N=445)Gastrointestinal Disorders Nausea 9 6 7 3 Dry Mouth 7 2 4 <1 Nervous System Disorders Headache 23 14 17 9 Psychiatric Disorders Insomnia 6 4 5 1 Depression 3 1 2 <1 Skin And Subcutaneous Tissue Disorders Rash 4 1 2 <1 ª Four double-blind, placebo-controlled clinical studies in SWD, OSA, and narcolepsy. Vital Sign Changes
There were small, but consistent, increases in average values for mean systolic and diastolic blood pressure in controlled trials (SeePRECAUTIONS). There was a small, but consistent, average increase in pulse rate over placebo in controlled trials. This increase varied from 0.9 to 3.5 BPM.
Laboratory Changes
Clinical chemistry, hematology, and urinalysis parameters were monitored in the studies. Mean plasma levels of gamma glutamyltransferase (GGT) and alkaline phosphatase (AP) were found to be higher following administration of NUVIGIL, but not placebo. Few subjects, however, had GGT or AP elevations outside of the normal range. No differences were apparent in alanine aminotransferase, aspartate aminotransferase, total protein, albumin, or total bilirubin, although there were rare cases of isolated elevations of AST and/or ALT. A single case of mild pancytopenia was observed after 35-days of treatment and resolved with drug discontinuation. A small mean decrease from baseline in serum uric acid compared to placebo was seen in clinical trials. The clinical significance of this finding is unknown.
-
DRUG ABUSE AND DEPENDENCE
Abuse Potential and Dependence
Although the abuse potential of armodafinil has not been specifically studied, its abuse potential is likely to be similar to that of modafinil (PROVIGIL). In humans, modafinil produces psychoactive and euphoric effects, alterations in mood, perception, thinking and feelings typical of other CNS stimulants. In in vitro binding studies, modafinil binds to the dopamine reuptake site and causes an increase in extracellular dopamine, but no increase in dopamine release. Modafinil is reinforcing, as evidenced by its self-administration in monkeys previously trained to self-administer cocaine. In some studies, modafinil was also partially discriminated as stimulant-like. Physicians should follow patients closely, especially those with a history of drug and/or stimulant (e.g., methylphenidate, amphetamine, or cocaine) abuse. Patients should be observed for signs of misuse or abuse (e.g., incrementation of doses or drug-seeking behavior).
The abuse potential of modafinil (200, 400, and 800 mg) was assessed relative to methylphenidate (45 and 90 mg) in an inpatient study in individuals experienced with drugs of abuse. Results from this clinical study demonstrated that modafinil produced psychoactive and euphoric effects and feelings consistent with other scheduled CNS stimulants (methylphenidate).
-
OVERDOSAGE
Human Experience
There were no overdoses reported in the NUVIGIL clinical studies. Symptoms of NUVIGIL overdose are likely to be similar to those of modafinil. Overdose in modafinil clinical trials included excitation or agitation, insomnia, and slight or moderate elevations in hemodynamic parameters. From post-marketing experience with modafinil, there have been no reports of fatal overdoses involving modafinil alone (doses up to 12 grams). Overdoses involving multiple drugs, including modafinil, have resulted in fatal outcomes. Symptoms most often accompanying modafinil overdose, alone or in combination with other drugs have included; insomnia; central nervous system symptoms such as restlessness, disorientation, confusion, excitation and hallucination; digestive changes such as nausea and diarrhea; and cardiovascular changes such as tachycardia, bradycardia, hypertension and chest pain.
Overdose Management
No specific antidote exists for the toxic effects of a NUVIGIL overdose. Such overdoses should be managed with primarily supportive care, including cardiovascular monitoring. If there are no contraindications, induced emesis or gastric lavage should be considered. There are no data to suggest the utility of dialysis or urinary acidification or alkalinization in enhancing drug elimination. The physician should consider contacting a poison-control center for advice in the treatment of any overdose.
-
DOSAGE AND ADMINISTRATION
Obstructive Sleep Apnea (OSA) and Narcolepsy
The recommended dose of NUVIGIL for patients with OSA or narcolepsy is 150 mg or 250 mg given as a single dose in the morning. In patients with OSA, doses up to 250 mg/day, given as a single dose, have been well tolerated, but there is no consistent evidence that this dose confers additional benefit beyond that of the 150 mg/day dose (See CLINICAL PHARMACOLOGY andCLINICAL TRIALS).
Shift Work Sleep Disorder (SWD)
The recommended dose of NUVIGIL for patients with SWD is 150 mg given daily approximately 1 hour prior to the start of their work shift.
Dosage adjustment should be considered for concomitant medications that are substrates for CYP3A4/5, such as steroidal contraceptives, triazolam, and cyclosporine (See PRECAUTIONS, Drug Interactions).
Drugs that are largely eliminated via CYP2C19 metabolism, such as diazepam, propranolol, and phenytoin may have prolonged elimination upon coadministration with NUVIGIL and may require dosage reduction and monitoring for toxicity (See PRECAUTIONS, Drug Interactions).
In patients with severe hepatic impairment, NUVIGIL should be administered at a reduced dose (See CLINICAL PHARMACOLOGYandPRECAUTIONS).
There is inadequate information to determine safety and efficacy of dosing in patients with severe renal impairment (SeeCLINICAL PHARMACOLOGYandPRECAUTIONS).
In elderly patients, elimination of armodafinil and its metabolites may be reduced as a consequence of aging. Therefore, consideration should be given to the use of lower doses in this population (See CLINICAL PHARMACOLOGY andPRECAUTIONS).
-
HOW SUPPLIED
NUVIGIL® (armodafinil) Tablets [C-IV]
150 mg:Each oval, white uncoated tablet is debossed with "C" on one side and "215" on the other.
NDC: 16590-345-30 - Bottles of 30
250 mg: Each oval, white uncoated tablet is debossed with "C" on one side and "225" on the other.
NDC: 16590-394-30 - Bottles of 30
Store at 20° - 25° C (68° - 77° F).
Distributed by:
Cephalon, Inc.
Frazer, PA 19355
U.S. Patent Nos. RE37,516; 7,132,570; 7,297,346
NUVIGIL is a trademark of Cephalon, Inc. or its affiliates.
© 2007-2010 Cephalon, Inc. All rights reserved.
October 2010
NUV-006
Relabeling and Repackaging by:
STAT Rx USA LLC
Gainesville, GA 30501 -
Medication Guide
NUVIGIL (nu-vij-el) C-IV
(armodafinil)
Tablets
Read the Medication Guide that comes with NUVIGIL before you start taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking with your doctor about your condition or treatment.
What is the most important information I should know about NUVIGIL?
NUVIGIL may cause serious side effects including a serious rash or a serious allergic reaction that may affect parts of your body such as your liver or blood cells. Any of these may need to be treated in a hospital and may be life-threatening.
Stop taking NUVIGIL and call your doctor right away or get emergency help if you have any of these symptoms:
-
- skin rash, hives, sores in your mouth, or your skin blisters and peels
- swelling of your face, eyes, lips, tongue, or throat
- trouble swallowing or breathing
- fever, shortness of breath, swelling of the legs, yellowing of the skin or whites of the eyes, or dark urine.
If you have a severe rash with NUVIGIL, stopping the medicine may not keep the rash from becoming life-threatening or causing you to be permanently disabled or disfigured.
NUVIGIL is not approved for use in children for any medical condition.
It is not known if NUVIGIL is safe or if it works in children under the age of 17.
What is NUVIGIL?
NUVIGIL is a prescription medicine used to improve wakefulness in adults who are very sleepy due to one of the following diagnosed sleep disorders:
- narcolepsy
- obstructive sleep apnea (OSA). NUVIGIL is used with other medical treatments for this sleep disorder. NUVIGIL does not take the place of using your CPAP machine or other treatments that your doctor has prescribed for this condition. It is important that you continue to use these treatments as prescribed by your doctor.
- shift work disorder (SWD)
NUVIGIL will not cure these sleep disorders. NUVIGIL may help the sleepiness caused by these conditions, but it may not stop all your sleepiness. NUVIGIL does not take the place of getting enough sleep. Follow your doctor's advice about good sleep habits and using other treatments.

Who should not take NUVIGIL?
Do not take NUVIGIL if you:
- are allergic to any of its ingredients. See the end of this Medication Guide for a complete list of ingredients in NUVIGIL.
- have had a rash or allergic reaction to either armodafinil (NUVIGIL) or modafinil (PROVIGIL®). These medicines are very similar.
What should I tell my doctor before taking NUVIGIL?
Tell your doctor about all of your medical conditions including, if you:
- have a history of mental health problems, including psychosis
- have heart problems or had a heart attack
- have high blood pressure. Your blood pressure may need to be checked more often while taking NUVIGIL.
- have liver or kidney problems
- have a history of drug or alcohol abuse or addiction
- are pregnant or planning to become pregnant. It is not known if NUVIGIL will harm your unborn baby.
Pregnancy Registry: There is a registry for women who become pregnant during treatment with NUVIGIL. The purpose of this registry is to collect information about the safety of NUVIGIL during pregnancy. Contact the registry as soon as you learn that you are pregnant, or ask your doctor to contact the registry for you. You or your doctor can get information and enroll you in the registry by calling 1-866-404-4106.
- are breastfeeding. It is not known if NUVIGIL passes into your milk. Talk to your doctor about the best way to feed your baby if you take NUVIGIL.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. NUVIGIL and many other medicines can interact with each other, sometimes causing side effects. NUVIGIL may affect the way other medicines work, and other medicines may affect how NUVIGIL works. Your dose of NUVIGIL or certain other medicines may need to be changed.
Especially, tell your doctor if you use or take:
- a hormonal birth control method, such as birth control pills, shots, implants, patches, vaginal rings, and intrauterine devices (IUDs). Hormonal birth control methods may not work while you take NUVIGIL. Women who use one of these methods of birth control may have a higher chance for getting pregnant while taking NUVIGIL, and for one month after stopping NUVIGIL. Talk to your doctor about birth control choices that are right for you while taking NUVIGIL.
Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine. Your doctor or pharmacist will tell you if it is safe to take NUVIGIL and other medicines together. Do not start any new medicines with NUVIGIL unless your doctor has told you it is okay.
How should I take NUVIGIL?
- Take NUVIGIL exactly as prescribed by your doctor. Your doctor will prescribe the dose of NUVIGIL that is right for you. Do not change your dose of NUVIGIL without talking to your doctor.
- Your doctor will tell you the right time of day to take NUVIGIL.
- People with narcolepsy or OSA usually take NUVIGIL one time each day in the morning.
- People with SWD usually take NUVIGIL about 1 hour before their work shift.
- Do not change the time of day you take NUVIGIL unless you have talked to your doctor. If you take NUVIGIL too close to your bedtime, you may find it harder to go to sleep.
- You can take NUVIGIL with or without food.
- If you take more than your prescribed dose or if you take an overdose of NUVIGIL, call your doctor or poison control center right away.
Symptoms of an overdose of NUVIGIL may include:
- Trouble sleeping
- Restlessness
- Confusion
- Feeling disoriented
- Feeling excited
- Hearing, seeing, feeling, or sensing things that are not really there (hallucinations)
- Nausea and diarrhea
- A fast or slow heartbeat
- Chest pain
- Increased blood pressure
What should I avoid while taking NUVIGIL?
- Do not drive a car or do other dangerous activities until you know how NUVIGIL affects you. People with sleep disorders should always be careful about doing things that could be dangerous. Do not change your daily habits until your doctor tells you it is okay.
- You should avoid drinking alcohol. It is not known how drinking alcohol will affect you when taking NUVIGIL.
What are possible side effects of NUVIGIL?
NUVIGIL may cause serious side effects. Stop taking NUVIGIL and call your doctor right away or get emergency help if you get any of the following:
- a serious rash or serious allergic reaction. (See “What is the most important information I should know about NUVIGIL?”)
-
mental (psychiatric) symptoms, including:
- depression
- feeling anxious
- hearing, seeing, feeling, or sensing things that are not really there (hallucinations)
- an extreme increase in activity and talking (mania)
- thoughts of suicide
- aggressive behavior
- other mental problems
- symptoms of a heart problem, including chest pain, abnormal heart beats, and trouble breathing.
Common side effects that can happen in anyone who takes NUVIGIL include:
- headache
- nausea
- dizziness
- trouble sleeping
Tell your doctor if you get any side effect that bothers you or that does not go away while taking NUVIGIL.
These are not all the side effects of NUVIGIL. For more information, ask your doctor or pharmacist.
Some effects of NUVIGIL on the brain are the same as other medicines called “stimulants”. These effects may lead to abuse or dependence on NUVIGIL.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store NUVIGIL?
- Store NUVIGIL at room temperature between 68° and 77° F (20° and 25° C).
- Keep NUVIGIL and all medicines out of the reach of children.
General information about NUVIGIL
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use NUVIGIL for a condition for which it was not prescribed. Do not give NUVIGIL to other people, even if they have the same symptoms you have. It may harm them and it is against the law.
This Medication Guide summarizes the most important information about NUVIGIL. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about NUVIGIL that is written for health professionals. For more information, call 1-800-896-5855, or go to www.NUVIGIL.com.
What are the ingredients in NUVIGIL?
Active Ingredient: armodafinil
Inactive Ingredients: lactose monohydrate, microcrystalline cellulose, pregelatinized starch, croscarmellose sodium, povidone, and magnesium stearate.
Distributed by:
Cephalon, Inc.
Frazer, PA 19355
NUVMG – 001
Revised October 2010
This Medication Guide has been approved by the U.S. Food and Drug Administration.
© 2007 - 2010 Cephalon, Inc. All rights reserved.
-
- SPL UNCLASSIFIED SECTION
- PACKAGE LABEL - NUVIGIL C-IV 150 MG TABLETS
- PACKAGE LABEL - NUVIGIL C-IV 250 MG TABLETS
-
INGREDIENTS AND APPEARANCE
NUVIGIL
armodafinil tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 16590-345(NDC:63459-215) Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARMODAFINIL (UNII: V63XWA605I) (ARMODAFINIL - UNII:V63XWA605I) ARMODAFINIL 150 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color WHITE (White to off-white) Score no score Shape OVAL Size 13mm Flavor Imprint Code 215 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 16590-345-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021875 06/01/2009 NUVIGIL
armodafinil tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 16590-394(NDC:63459-225) Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARMODAFINIL (UNII: V63XWA605I) (ARMODAFINIL - UNII:V63XWA605I) ARMODAFINIL 250 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color WHITE (White to off-white) Score no score Shape OVAL Size 16mm Flavor Imprint Code 225 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 16590-394-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021875 06/01/2009 Labeler - STAT Rx USA LLC (786036330) Registrant - PSS World Medical Inc. (101822682) Establishment Name Address ID/FEI Business Operations STAT Rx USA LLC 786036330 relabel(16590-345, 16590-394) , repack(16590-345, 16590-394)
Trademark Results [NUVIGIL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NUVIGIL 78426061 3538564 Live/Registered |
Cephalon, Inc. 2004-05-27 |
 NUVIGIL 77668359 3782440 Live/Registered |
Cephalon, Inc. 2009-02-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

