PROFILNINE- factor ix complex kit
Profilnine by
Drug Labeling and Warnings
Profilnine by is a Other medication manufactured, distributed, or labeled by GRIFOLS USA, LLC, Grifols Biologicals LLC, LABORATORIOS GRIFOLS SA, GRIFOLS BIOLOGICALS LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Profilnine®, Factor IX Complex, is a solvent/detergent treated, nanofiltered, sterile, lyophilized concentrate of coagulation factors IX, II, X, and low levels of factor VII. The factor II content is not more than (NMT) 150 units* per 100 factor IX units, the factor X content is NMT 100 units per 100 factor IX units, and the factor VII content is NMT 35 units per 100 factor IX units. Profilnine does not contain heparin and contains no preservatives. Profilnine contains few, if any, activated factors based on results from the non-activated partial thromboplastin time (NAPTT) test1,2.
Profilnine is intended for intravenous administration only. Each vial is a single-dose container and is labeled with the factor IX potency expressed in International Units.
Profilnine is prepared from pooled human plasma and purified by diethylaminoethyl (DEAE) cellulose adsorption. The risk of transmission of infective agents by Profilnine has been substantially reduced by donor selection procedures and virus screening of individual donations and plasma pools by serological and nucleic acid testing. In addition, virus elimination steps such as nanofiltration3 and solvent/detergent (tri-n-butyl phosphate) treatment4 have been incorporated into the Profilnine manufacturing process. Additional removal of some viruses occurs during the DEAE cellulose product purification step.
The ability of the manufacturing process to eliminate virus from Profilnine was evaluated in the laboratory by intentionally adding virus to product just prior to the elimination step and monitoring virus removal. Table 1 shows the amounts of virus that can be removed by solvent/detergent treatment, nanofiltration, and purification by DEAE chromatography when vesicular stomatitis virus (VSV), human immunodeficiency virus-1 and 2 (HIV-1, HIV-2), parvovirus, West Nile virus (WNV), bovine viral diarrhea virus (BVDV), hepatitis A virus (HAV), and pseudorabies virus (PRV) were evaluated in these virus spiking studies. The results indicate that the solvent/detergent treatment step inactivates enveloped viruses and the nanofiltration step removes both enveloped and non-enveloped viruses.
___________________________________
* Unit refers to International Unit in the labeling of Profilnine.Table 1: Virus Reduction * Porcine, NT=Not tested, Env=Enveloped
Virus
Virus
Type
Model
For:Virus Reduction (log10) Process Step 1st DEAE Chromatography Solvent-Detergent Nanofiltration Sindbis Env Hepatitis C 1.4 ≥ 5.3 NT VSV Env Robust enveloped viruses NT ≥ 4.9 NT HIV-1 Env HIV-1 NT ≥ 12.2 ≥ 6.2 HIV-2 Env HIV-2 NT ≥ 6.0 NT WNV Env WNV NT NT ≥ 6.6 BVDV Env Hepatitis C NT NT ≥ 4.9 Parvo* Non-Env Parvovirus B19 NT NT ≥ 6.1 HAV Non-Env HAV NT NT ≥ 5.8 PRV Env Hepatitis B NT NT ≥ 5.3 -
CLINICAL PHARMACOLOGY
Profilnine is a mixture of the vitamin K-dependent clotting factors IX, II, X, and low levels of VII. The administration of Profilnine temporarily increases the plasma levels of factor IX, thus enabling a temporary correction of the factor deficiency.
A clinical study that evaluated twelve subjects with hemophilia B indicated that, following administration of Profilnine, the factor IX in vivo half-life was 24.68 ± 8.29 hours and recovery was 1.15 ± 0.16 units/dL per unit infused per kg body weight.
Administration of Factor IX Complex can result in higher than normal levels of factor II due to the significantly longer half-life of factor II5.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Transmissible Infectious Agents
Because Profilnine is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent.
Inhibitors
Patients can develop neutralizing antibodies (inhibitors) after treatment with Profilnine. Monitor patients for inhibitors, which should be quantified in Bethesda Units (BU) using appropriate laboratory testing.
Hypersensitivity
Hypersensitivity, including anaphylaxis, has been reported. Inform patients of the early symptoms and signs of hypersensitivity reaction, including hives, generalized urticaria, angioedema, chest tightness, dyspnea, wheezing, faintness, hypotension, tachycardia, and anaphylaxis.
Thrombosis
The use of factor IX complex concentrates has been associated with the development of thromboembolic complications. Patients at increased risk for thrombosis include those undergoing surgery, post surgery, with known liver disease, and with signs of fibrinolysis, thrombosis, or disseminated intravascular coagulation (DIC)5. When administering Profilnine to these high-risk patients, monitor for early signs of consumptive coagulopathy with appropriate laboratory testing. Only administer Profilnine to patients when the benefits outweigh the risks.
-
PRECAUTIONS
Vasomotor reactions may result from overly rapid administration. Do not exceed the recommended infusion rate of 10 mL/min.
Information for Patients
Advise patients to report to their physician any decrease in effectiveness of Factor IX treatment, as this can indicate development of inhibitors.
Hypersensitivity, including anaphylaxis, has been reported for factor IX complex concentrate products. Inform patients of the early symptoms and signs of hypersensitivity reaction, including hives, rash, swelling, chest tightness, shortness of breath, wheezing, faintness, decrease in blood pressure, and rapid heartbeat. Advise patients to discontinue use of the product and contact their physician and/or seek immediate emergency care if these symptoms occur.
-
ADVERSE REACTIONS
Adverse reactions with Profilnine may include headache, fever, chills, flushing, nausea, vomiting, tingling, lethargy, urticaria, and manifestations of allergic reactions.
The following adverse reactions have been identified during post-approval use of Profilnine: hypersensitivity reactions including shortness of breath, diaphoresis, and hypotension, as well as thrombosis including pulmonary embolism and deep vein thrombosis, disseminated intravascular coagulation, and inhibitor development. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
To report SUSPECTED ADVERSE REACTIONS, contact Grifols at 1-888-GRIFOLS (1-888-474-3657) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
DOSAGE AND ADMINISTRATION
Dose
Each vial of Profilnine is labeled with total units expressed as International Units (IU). According to the WHO International Standard, one unit approximates the activity in one mL of normal plasma.
A 1% increase in factor IX (0.01 units) per unit administered per kg body weight can be expected1. The amount of Profilnine required to establish hemostasis will vary with each patient and circumstance. Use the following formula and example as guides in determining the number of units to be administered:
Body weight (in kg) X Desired increase in Plasma Factor IX (Percent) X 1 Units/kg = Number of Factor IX Units Required Example: 50 kg X 25 (% increase) X 1 Units/kg = 1,250 Units of factor IX Due to variability among patients and their clinical condition, monitor the factor IX level of each patient frequently during replacement therapy.
Table 2 below provides treatment guidelines for hemorrhagic events and surgery in patients with factor IX deficiency.
Table 2: Treatment Guidelines Type of Bleeding or Surgical Procedure Factor IX Level Required, % of Normal (Dose) Frequency of Doses Duration of Therapy (Days) Minor to Moderate Hemorrhages 20-30% (20-30 IU FIX/kg) until hemorrhage stops and healing has been achieved.
Every 16-24 hrs Minor: 1-2 days
Moderate: 2-7 daysMajor Hemorrhages 30-50% (30-50 IU FIX/kg).
Following this treatment period, maintain FIX levels at 20% (20 IU FIX/kg) until healing has been achieved.Every 16-24 hrs 3-10 days Surgery Prior to surgery,
30-50% (30-50 IU FIX/kg).
For dental extractions, bring FIX levels to 50% immediately prior to the procedure.
Maintain FIX levels at 30-50% (30-50 IU FIX/kg) until healing has been achieved.Every 16-24 hrs 7-10 days Dosing requirements and frequency of dosing are calculated on the basis of an initial response of 1% FIX increase achieved per IU of FIX infused per kg body weight and an average half-life for FIX of 24 hours. If dosing studies reveal that a particular patient exhibits a lower response, monitor blood levels and adjust the dose accordingly.
Reconstitution
Use Aseptic Technique
- Ensure that concentrate (Profilnine) and diluent (Sterile Water for Injection, USP) are at room temperature (but not above 37° C) before reconstitution.
- Remove the plastic flip off cap from the diluent vial.
- Gently swab the exposed stopper surface with a cleansing agent such as alcohol. Avoid leaving any excess cleansing agent on the stopper.
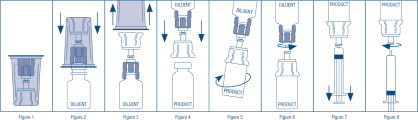
- Open the Mix2Vial® package by peeling away the lid (Figure 1). Leave the Mix2Vial in the clear outer packaging.
- Place the diluent vial upright on an even surface, hold the vial tightly, and pick up the Mix2Vial in its clear outer packaging. While holding the diluent vial securely, push the blue end of the Mix2Vial vertically down through the diluent vial stopper (Figure 2).
- While holding onto the diluent vial, carefully remove the clear outer packaging from the Mix2Vial set, ensuring the Mix2Vial remains attached to the diluent vial (Figure 3).
- Place the product vial upright on an even surface, invert the diluent vial with the Mix2Vial attached.
- While holding the product vial securely on a flat surface, push the clear end of the Mix2Vial set vertically down through the product vial stopper (Figure 4). The diluent will automatically transfer out of its vial into the product vial.
NOTE: If the Mix2Vial is connected at an angle, the vacuum may be released from the product vial and the diluent will not transfer into the product vial. - With the diluent and product vials still attached to the Mix2Vial, gently swirl the product vial to ensure the product is fully dissolved (Figure 5). Reconstitution requires less than 10 minutes. Do not shake the vial.
- Disconnect the Mix2Vial into two separate pieces (Figure 6) by holding each vial adapter and twisting counterclockwise. After separating, discard the diluent vial with the blue end of the Mix2Vial.
- Draw air into an empty, sterile syringe. Keeping the product vial upright with the clear end of the Mix2Vial attached, screw the disposable syringe onto the luer lock portion of the Mix2Vial device by pressing and twisting clockwise. Inject air into the product vial.
- While keeping the syringe plunger depressed, invert the system upside down and draw the reconstituted product into the syringe by pulling the plunger back slowly (Figure 7).
- When the reconstituted product has been transferred into the syringe, firmly hold the barrel of the syringe and the clear vial adapter (keeping the syringe plunger facing down) and unscrew the syringe from the Mix2Vial (Figure 8). Hold the syringe upright and push the plunger until no air is left in the syringe. Attach the syringe to a venipuncture set.
NOTE: If the same patient is to receive more than one vial of concentrate, the contents of two vials may be drawn into the same syringe through a separate unused Mix2Vial set before attaching to the venipuncture set. - After reconstitution, inspect parenteral drug products visually for particulate matter and discoloration prior to administration, whenever solution and container permit. When reconstitution procedure is strictly followed, a few small particles may occasionally remain. The Mix2Vial set will remove particles and the labeled potency will not be reduced.
- Do not refrigerate after reconstitution. The reconstituted product is stable for 3 hours at room temperature; use as soon as possible within 3 hours after reconstitution.
-
Administration
For intravenous administration only,
- Inspect the final solution visually for particulate matter and discoloration prior to administration.
- Administer the prepared drug at room temperature within three hours after reconstitution. Prompt administration is recommended to avoid ill effects of any inadvertent bacterial contamination occurring during reconstitution.
- Administer by intravenous injection (plastic disposable syringe only) or infusion at a rate not exceeding 10 mL/minute.
- Discard any unused Profilnine vial contents. Discard administration equipment into the appropriate safety container after single use. Do not resterilize components. Do not reuse components.
-
HOW SUPPLIED
Profilnine is supplied in sterile lyophilized form in single-dose vials accompanied by a suitable volume of diluent (Sterile Water for Injection, USP), according to factor IX potency. Each vial is labeled with the factor IX potency expressed in International Units which is referenced to the WHO International Standard. Profilnine is packaged with a Mix2Vial filter transfer set for use in administration.
The product is available in several potencies, with carton and vial label color coded based upon assay as follows:
Potency Carton NDC Assay Color Code 500 units FIX/5 mL 68516-3201-1 or 68516-3207-1 500 units FIX Range - blue 1000 units FIX/10 mL 68516-3202-2 or 68516-3208-2 1000 units FIX Range - red 1500 units FIX/10 mL 68516-3203-2 or 68516-3209-2 1500 units FIX Range - black The diluent vial stopper contains natural rubber latex. All other components of the kit are not made with natural rubber latex.
-
REFERENCES
- Menache, D., Roberts, H.R. Summary report and recommendations of the task force members and consultants. Thromb Diath Haemorrh 33:645-647, 1975.
- Kingdon, H.S., Lundblad, R.L., Veltkamp, J.J., Aronson, D.L. Potentially thrombogenic materials in Factor IX Concentrates. Thromb Diath Haemorrh 33:617- 631, 1975.
- Burnouf T, Radosevich M. Nanofiltration of plasma-derived biopharmaceutical products. Haemophilia : the official journal of the World Federation of Hemophilia. 2003;9:24-37.
- Dichtelmüller HO, Biesert L, Fabbrizzi F, Gajardo R, Gröner A, von Hoegen I, Jorquera JI, Kempf C, Kreil TR, Pifat D, Osheroff W, Poelsler G. Robustness of solvent/detergent treatment of plasma derivatives: a data collection from Plasma Protein Therapeutics Association member companies. Transfusion 49:1931-1943, 2009.
- Sorensen, B., Spahn, D.R., Innerhofer, P., Spannagl, M., Rossaint, R. Clinical review: prothrombin complex concentrates-evaluation of safety and thrombogenicity. Critical Care 15: 201-209, 2011.
Manufactured by:
Grifols Biologicals LLC
5555 Valley Boulevard
Los Angeles, CA 90032, U.S.A.
U.S. License No. 1694
DATE OF REVISION: June 20183051561
-
Principal Display Panel – 500 IU Vial Label
GRIFOLS
NDC: 68516-3204-1
Factor IX Complex
Profilnine®500 IU FIX Range
5 mL
Storage: Store at temperatures not exceeding 25 °C (77 °F)
Rx Only. Single dose container for intravenous
administration only.Grifols Biologicals LLC
5555 Valley Boulevard, Los Angeles, CA 90032, U.S.A.
U.S. License No. 1694Instructions: Reconstitute with 5 mL Sterile Water for Injection, USP.
Administer intravenously at room temperature within three hours of reconstitution.
Discard unused contents.
Contains Factors II, IX, X, and low levels of Factor VII.
Contains no preservatives.
For information on dosage and directions for administration, see accompanying pamphlet.
The patient and physician should discuss the risks and benefits of this product.
3051562
Lot
EXP
IU FIX/Vial
Lot
IU FIX/Vial
Profilnine® 5 mL NDC: 68516-3204-1

-
Principal Display Panel – 1000 IU Vial Label
GRIFOLS
NDC: 68516-3205-2
Factor IX Complex
Profilnine®1000 IU FIX Range
10 mL
Storage: Store at temperatures not exceeding 25 °C (77 °F).
Rx Only. Single dose container for intravenous
administration only.Grifols Biologicals LLC
5555 Valley Boulevard, Los Angeles, CA 90032, U.S.A.
U.S. License No. 1694
Instructions: Reconstitute with 10 mL Sterile Water for Injection, USP.
Administer intravenously at room temperature within three hours of reconstitution.
Discard unused contents.
Contains Factors II, IX, X, and low levels of Factor VII.
Contains no preservatives.
For information on dosage and directions for administration, see accompanying pamphlet.
The patient and physician should discuss the risks and benefits of this product.
3051565
Lot
EXP
IU FIX/Vial
Lot
IU FIX/Vial
Profilnine® 10 mL NDC: 68516-3205-2

-
Principal Display Panel – 1500 IU Vial Label
GRIFOLS
NDC: 68516-3206-2
Factor IX Complex
Profilnine®1500 IU FIX Range
10 mL
Storage: Store at temperatures not exceeding 25 °C (77 °F).
Rx Only. Single dose container for intravenous
administration only.Grifols Biologicals LLC
5555 Valley Boulevard, Los Angeles, CA 90032, U.S.A.
U.S. License No. 1694Instructions: Reconstitute with 10 mL Sterile Water for Injection, USP.
Administer intravenously at room temperature within three hours of reconstitution.
Discard unused contents.
Contains Factors II, IX, X, and low levels of Factor VII.
Contains no preservatives.
For information on dosage and directions for administration, see accompanying pamphlet.
The patient and physician should discuss the risks and benefits of this product.
3051568
Lot
EXP
IU FIX/Vial
Lot
IU FIX/Vial
Profilnine® 10 mL NDC: 68516-3206-2
-
Principal Display Panel - 5 mL Vial Label
NDC: 63323-185-05 18505
STERILE
WATERFOR INJECTION, USP
FOR DRUG DILUENT USE ONLY
Rx only
5 mL Single Dose Vial
Sterile, Nonpyrogenic
Preservative Free
Discard unused portion.
Warning: Do not administer intravenously unless rendered nearly isotonic.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
APP Pharmaceuticals, LLC
Schaumburg, IL 60173
401745D
LOT/EXP
-
Principal Display Panel - 10 mL Vial Label
NDC: 63323-185-10 918510
STERILE WATER
FOR INJECTION, USP
FOR DRUG DILUENT USE ONLY
Rx only
10 mL Single Dose Vial
Sterile, Nonpyrogenic
Preservative Free
Discard unused portion.
Do no administer intravenously unless rendered nearly isotonic.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
APP Pharmaceuticals, LLC
Schaumburg, IL 60173
401753E
LOT/EXP
-
Principal Display Panel 500 IU Carton Label
GRIFOLS
NDC: 68516-3207-1
Factor IX Complex
Profilnine®
5 mL
500 IU FIX Range
For Intravenous Administration
Contents: One vial Factor IX Complex, Profilnine®, one vial 5 mL Sterile Water for Injection, USP, one Mix2Vial® filter transfer set, and directions for use. Contains Factors II, IX, X and low levels of Factor VII.
The reconstituted product contains not more than 2.5 µg polysorbate 80 and 0.40 µg tri (n-butyl) phosphate per IU of Factor IX.
Contains no preservatives.
Administer within three hours of reconstitution.
Warning: This product is prepared from large pools of human plasma. Human blood and its components may transmit infectious agents. See package insert, WARNINGS AND PRECAUTIONS.
Instructions: The patient and physician should discuss the risks and benefits of this product. For information on dosage and directions for administration, see enclosed package insert.
Storage: Store at temperatures not exceeding 25 °C (77 °F). Do not freeze.
Rx only
Grifols Biologicals LLC
5555 Valley Boulevard
Los Angeles, CA 90032, U.S.A.
U.S. License No. 1694
GTIN 00368516320716
LOT XXXXXXXXXX
EXP DDMMMYYYY
SN XXXXXXXX XXXXXXXX
IU FIX/Vial XXXX
3051564
-
Principal Display Panel - 5 mL Vial Label
NDC: 68516-1001-1
Sterile Water for Injection, USP
5 mL
Rx Only
For reconstitution of accompanying product
Single-Dose Container, Nonpyrogenic
Do not use unless clear.
No antimicrobial agent or other substance has been added.
Do not use for intravascular injection without making approximately isotonic by addition of suitable solute.
Discard unused portion.
Mfd by: Laboratorios Grifols, S. A. Parets del Vallès, Barcelona 08150 Spain
Mfd for: Grifols Biologicals LLC Los Angeles, CA 90032, USA
Lot
EXP
3051532

-
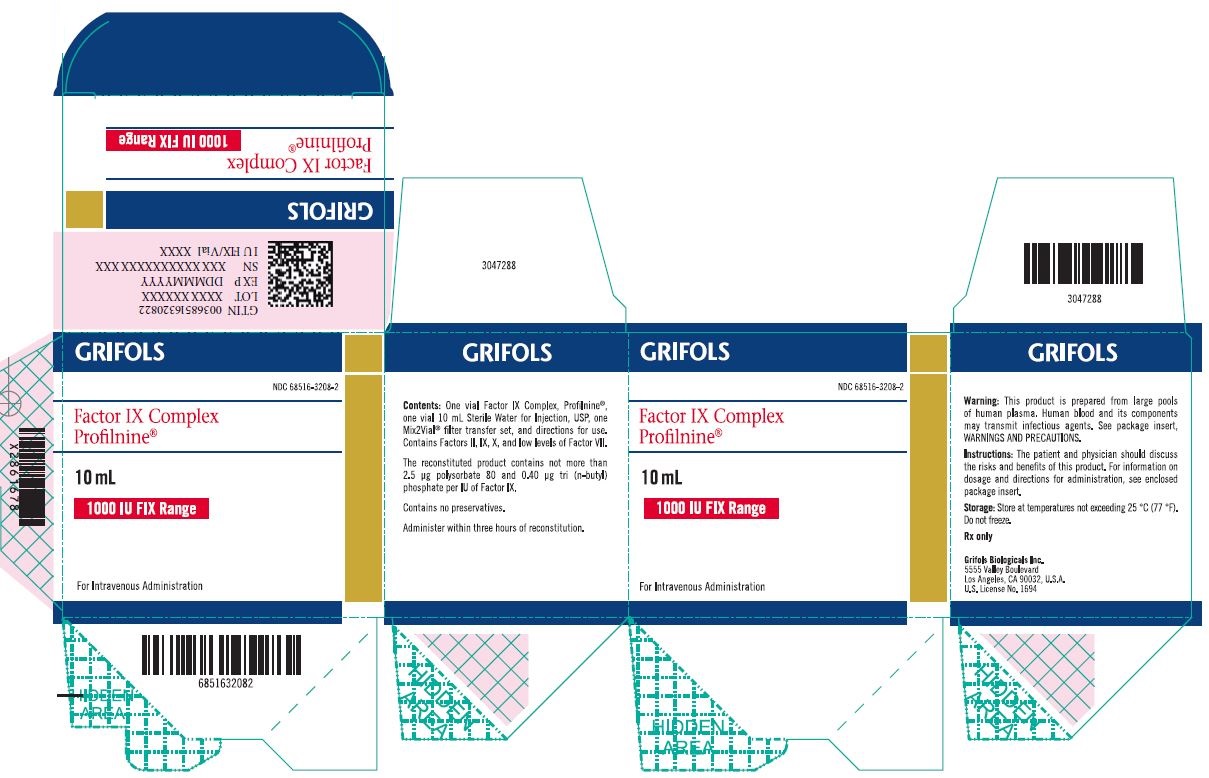
Principal Display Panel 1000 IU Carton Label
GRIFOLS
NDC: 68516-3208-2
Factor IX Complex
Profilnine®
10 mL
1000 IU FIX Range
For Intravenous Administration
Contents: One vial Factor IX Complex, Profilnine®, one vial 10 mL Sterile Water for Injection, USP, one Mix2Vial® filter transfer set, and directions for use. Contains Factors II, IX, X and low levels of Factor VII.
The reconstituted product contains not more than 2.5 µg polysorbate 80 and 0.40 µg tri (n-butyl) phosphate per IU of Factor IX.
Contains no preservatives.
Administer within three hours of reconstitution.
Warning: This product is prepared from large pools of human plasma. Human blood and its components may transmit infectious agents. See package insert, WARNINGS AND PRECAUTIONS.
Instructions: The patient and physician should discuss the risks and benefits of this product. For information on dosage and directions for administration, see enclosed package insert.
Storage: Store at temperatures not exceeding 25 °C (77 °F). Do not freeze.
Rx only
Grifols Biologicals LLC
5555 Valley Boulevard
Los Angeles, CA 90032, U.S.A.
U.S. License No. 1694
GTIN 00368516320822
LOT XXXXXXXXXX
EXP DDMMMYYYY
SN XXXXXXXX XXXXXXXX
IU FIX/Vial XXXX
3051567
-
Principal Display Panel - 10 mL Vial Label
NDC: 68516-1002-2
Sterile Water for Injection, USP
10 mL
Rx Only
For reconstitution of accompanying product
Single-Dose Container, Nonpyrogenic
Do not use unless clear.
No antimicrobial agent or other substance has been added.
Do not use for intravascular injection without making approximately isotonic by addition of suitable solute.
Discard unused portion.
Mfd by: Laboratorios Grifols, S. A. Parets del Vallès, Barcelona 08150 Spain
Mfd for: Grifols Biologicals LLC Los Angeles, CA 90032, USA
Lot
EXP
3051533

-
Principal Display Panel 1500 IU Carton Label
GRIFOLS
NDC: 68516-3209-2
Factor IX Complex
Profilnine®
10 mL
1500 IU FIX Range
For Intravenous Administration
Contents: One vial Factor IX Complex, Profilnine®, one vial 10 mL Sterile Water for Injection, USP, one Mix2Vial® filter transfer set, and directions for use. Contains Factors II, IX, X and low levels of Factor VII.
The reconstituted product contains not more than 2.5 µg polysorbate 80 and 0.40 µg tri (n-butyl) phosphate per IU of Factor IX.
Contains no preservatives.
Administer within three hours of reconstitution.
Warning: This product is prepared from large pools of human plasma. Human blood and its components may transmit infectious agents. See package insert, WARNINGS AND PRECAUTIONS.
Instructions: The patient and physician should discuss the risks and benefits of this product. For information on dosage and directions for administration, see enclosed package insert.
Storage: Store at temperatures not exceeding 25 °C (77 °F). Do not freeze.
Rx only
Grifols Biologicals LLC
5555 Valley Boulevard
Los Angeles, CA 90032, U.S.A.
U.S. License No. 1694
GTIN 00368516320921
LOT XXXXXXXXXX
EXP DDMMMYYYY
SN XXXXXXXX XXXXXXXX
IU FIX/Vial XXXX
3051570

-
INGREDIENTS AND APPEARANCE
PROFILNINE
factor ix complex kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 68516-3201 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68516-3201-1 1 in 1 CARTON; Type 6: Drug/Biologic Combination Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 5 mL Part 2 1 VIAL, SINGLE-DOSE 5 mL Part 1 of 2 PROFILNINE
factor ix complex injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 68516-3204 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength factor ix complex (UNII: FW411QXD5M) (factor ix complex - UNII:FW411QXD5M) factor ix complex 500 [iU] in 5 mL Inactive Ingredients Ingredient Name Strength sodium chloride (UNII: 451W47IQ8X) sodium citrate (UNII: 1Q73Q2JULR) sodium phosphate (UNII: SE337SVY37) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68516-3204-1 5 mL in 1 VIAL; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA102476 07/20/1981 Part 2 of 2 STERILE WATER
water injectionProduct Information Item Code (Source) NDC: 63323-185 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength water (UNII: 059QF0KO0R) (Water - UNII:059QF0KO0R) water 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63323-185-05 5 mL in 1 VIAL, SINGLE-DOSE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA088400 07/20/1981 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA102476 07/20/1981 10/31/2019 PROFILNINE
factor ix complex kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 68516-3207 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68516-3207-1 1 in 1 CARTON; Type 6: Drug/Biologic Combination Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 5 mL Part 2 1 VIAL, SINGLE-DOSE 5 mL Part 1 of 2 PROFILNINE
factor ix complex injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 68516-3204 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength factor ix complex (UNII: FW411QXD5M) (factor ix complex - UNII:FW411QXD5M) factor ix complex 500 [iU] in 5 mL Inactive Ingredients Ingredient Name Strength sodium chloride (UNII: 451W47IQ8X) sodium citrate (UNII: 1Q73Q2JULR) sodium phosphate (UNII: SE337SVY37) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68516-3204-1 5 mL in 1 VIAL; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA102476 07/20/1981 Part 2 of 2 STERILE WATER
water injectionProduct Information Item Code (Source) NDC: 68516-1001 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength water (UNII: 059QF0KO0R) (Water - UNII:059QF0KO0R) water 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68516-1001-1 5 mL in 1 VIAL, SINGLE-DOSE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA102476 07/20/1981 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA102476 07/20/1981 PROFILNINE
factor ix complex kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 68516-3202 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68516-3202-2 1 in 1 CARTON; Type 6: Drug/Biologic Combination Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 10 mL Part 2 1 VIAL, SINGLE-DOSE 10 mL Part 1 of 2 PROFILNINE
factor ix complex injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 68516-3205 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength factor ix complex (UNII: FW411QXD5M) (factor ix complex - UNII:FW411QXD5M) factor ix complex 100 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength sodium chloride (UNII: 451W47IQ8X) sodium citrate (UNII: 1Q73Q2JULR) sodium phosphate (UNII: SE337SVY37) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68516-3205-2 10 mL in 1 VIAL; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA102476 07/20/1981 Part 2 of 2 STERILE WATER
water injectionProduct Information Item Code (Source) NDC: 63323-185 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength water (UNII: 059QF0KO0R) (Water - UNII:059QF0KO0R) water 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63323-185-10 10 mL in 1 VIAL, SINGLE-DOSE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA088400 07/20/1981 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA102476 07/20/1981 03/31/2020 PROFILNINE
factor ix complex kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 68516-3208 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68516-3208-2 1 in 1 CARTON; Type 6: Drug/Biologic Combination Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 10 mL Part 2 1 VIAL, SINGLE-DOSE 10 mL Part 1 of 2 PROFILNINE
factor ix complex injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 68516-3205 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength factor ix complex (UNII: FW411QXD5M) (factor ix complex - UNII:FW411QXD5M) factor ix complex 100 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength sodium chloride (UNII: 451W47IQ8X) sodium citrate (UNII: 1Q73Q2JULR) sodium phosphate (UNII: SE337SVY37) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68516-3205-2 10 mL in 1 VIAL; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA102476 07/20/1981 Part 2 of 2 STERILE WATER
water injectionProduct Information Item Code (Source) NDC: 68516-1002 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength water (UNII: 059QF0KO0R) (Water - UNII:059QF0KO0R) water 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68516-1002-2 10 mL in 1 VIAL, SINGLE-DOSE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA102476 07/20/1981 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA102476 07/20/1981 PROFILNINE
factor ix complex kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 68516-3203 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68516-3203-2 1 in 1 CARTON; Type 6: Drug/Biologic Combination Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 10 mL Part 2 1 VIAL, SINGLE-DOSE 10 mL Part 1 of 2 PROFILNINE
factor ix complex injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 68516-3206 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength factor ix complex (UNII: FW411QXD5M) (factor ix complex - UNII:FW411QXD5M) factor ix complex 150 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength sodium chloride (UNII: 451W47IQ8X) sodium citrate (UNII: 1Q73Q2JULR) sodium phosphate (UNII: SE337SVY37) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68516-3206-2 10 mL in 1 VIAL; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA102476 07/20/1981 Part 2 of 2 STERILE WATER
water injectionProduct Information Item Code (Source) NDC: 63323-185 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength water (UNII: 059QF0KO0R) (Water - UNII:059QF0KO0R) water 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63323-185-10 10 mL in 1 VIAL, SINGLE-DOSE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA088400 07/20/1981 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA102476 07/20/1981 03/31/2020 PROFILNINE
factor ix complex kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 68516-3209 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68516-3209-2 1 in 1 CARTON; Type 6: Drug/Biologic Combination Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 10 mL Part 2 1 VIAL, SINGLE-DOSE 10 mL Part 1 of 2 PROFILNINE
factor ix complex injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 68516-3206 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength factor ix complex (UNII: FW411QXD5M) (factor ix complex - UNII:FW411QXD5M) factor ix complex 150 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength sodium chloride (UNII: 451W47IQ8X) sodium citrate (UNII: 1Q73Q2JULR) sodium phosphate (UNII: SE337SVY37) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68516-3206-2 10 mL in 1 VIAL; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA102476 07/20/1981 Part 2 of 2 STERILE WATER
water injectionProduct Information Item Code (Source) NDC: 68516-1002 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength water (UNII: 059QF0KO0R) (Water - UNII:059QF0KO0R) water 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68516-1002-2 10 mL in 1 VIAL, SINGLE-DOSE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA102476 07/20/1981 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA102476 07/20/1981 Labeler - GRIFOLS USA, LLC (048987452) Establishment Name Address ID/FEI Business Operations Grifols Biologicals LLC 092694538 manufacture(68516-3201, 68516-3202, 68516-3203, 68516-3204, 68516-3205, 68516-3206, 68516-3207, 68516-3208, 68516-3209) Establishment Name Address ID/FEI Business Operations Grifols Biologicals LLC 121076871 manufacture(68516-3201, 68516-3202, 68516-3203, 68516-3204, 68516-3205, 68516-3206, 68516-3207, 68516-3208, 68516-3209) Establishment Name Address ID/FEI Business Operations LABORATORIOS GRIFOLS SA 463719725 manufacture(68516-1001, 68516-1002) Establishment Name Address ID/FEI Business Operations Fresenius Kabi USA LLC 840771732 manufacture(63323-185)
Trademark Results [Profilnine]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PROFILNINE 73410690 1270548 Live/Registered |
Alpha Therapeutic Corporation 1983-01-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.