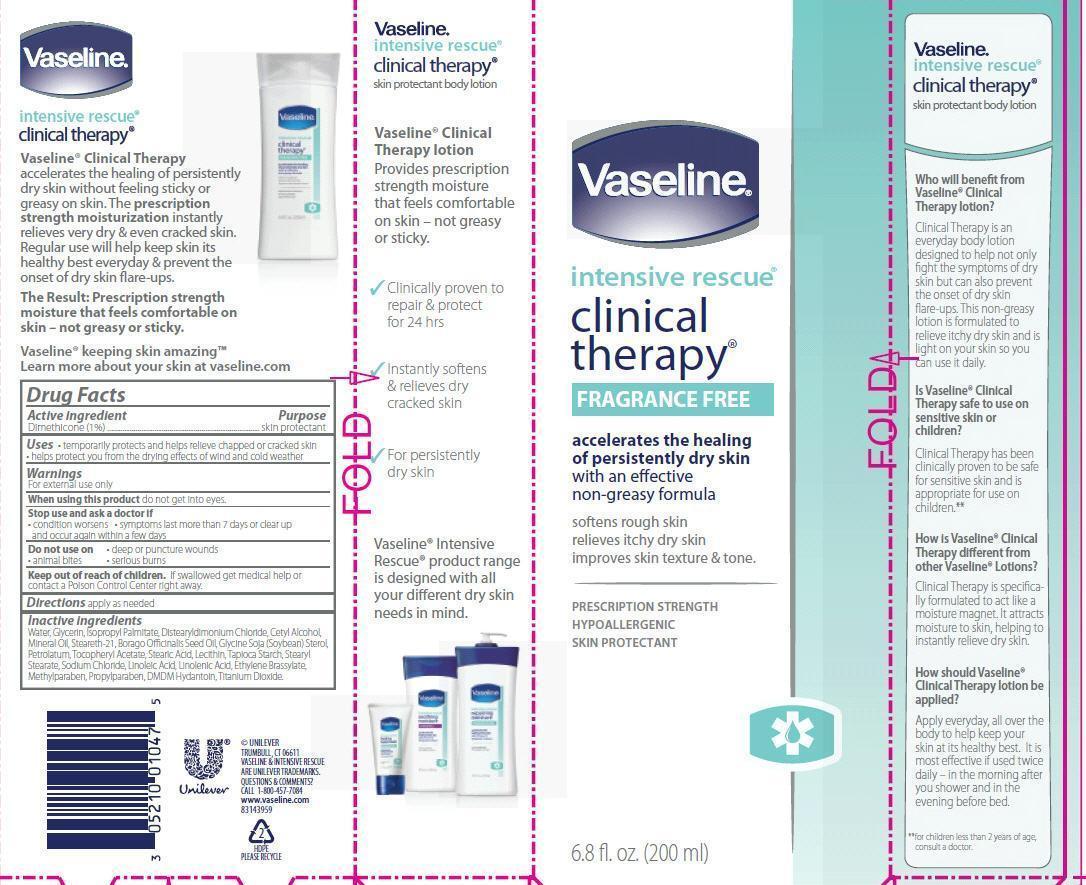
VASELINE INTENSIVE RESCUE CLINICAL THERAPY FRAGRANCE FREE- dimethicone lotion
Vaseline by
Drug Labeling and Warnings
Vaseline by is a Otc medication manufactured, distributed, or labeled by Conopco Inc. d/b/a Unilever. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive ingredients
Water, Glycerin, Isopropyl Palmitate, Distearyldimonium Chloride, Cetyl Alcohol, Mineral Oil, Steareth-21, Borago Officinalis Seed Oil, Glycine Soja (Soybean) Sterol, Petrolatum, Tocopheryl Acetate, Stearic Acid, Lecithin, Tapioca Starch, Stearyl Stearate, Sodium Chloride, Linoleic Acid, Linolenic Acid, Ethylene Brassylate, Methylparaben, Propylparaben, DMDM Hydantoin, Titanium Dioxide.
- QUESTIONS
- PDP 6.8 fl. oz.
- 6.8 fl. oz. carton
-
INGREDIENTS AND APPEARANCE
VASELINE INTENSIVE RESCUE CLINICAL THERAPY FRAGRANCE FREE
dimethicone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 64942-1038 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) CETYL ALCOHOL (UNII: 936JST6JCN) MINERAL OIL (UNII: T5L8T28FGP) STEARETH-21 (UNII: 53J3F32P58) BORAGO OFFICINALIS SEED (UNII: 2GXJ790US0) PETROLATUM (UNII: 4T6H12BN9U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) STEARIC ACID (UNII: 4ELV7Z65AP) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) STARCH, TAPIOCA (UNII: 24SC3U704I) STEARYL STEARATE (UNII: 5WX2EGD0DK) SODIUM CHLORIDE (UNII: 451W47IQ8X) LINOLEIC ACID (UNII: 9KJL21T0QJ) LINOLENIC ACID (UNII: 0RBV727H71) DMDM HYDANTOIN (UNII: BYR0546TOW) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 64942-1038-2 1 in 1 CARTON 1 NDC: 64942-1038-1 200 mL in 1 BOTTLE, PLASTIC 2 NDC: 64942-1038-3 50 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 06/23/2008 Labeler - Conopco Inc. d/b/a Unilever (001375088)
Trademark Results [Vaseline]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VASELINE 90186617 not registered Live/Pending |
Guo, Zhennan 2020-09-16 |
 VASELINE 85637053 4704115 Live/Registered |
Conopco, Inc. 2012-05-29 |
 VASELINE 85430833 4248617 Live/Registered |
CONOPCO, INC. 2011-09-23 |
 VASELINE 78943125 not registered Dead/Abandoned |
Unilever Supply Chain, Inc. 2006-08-02 |
 VASELINE 78735301 3295224 Dead/Cancelled |
CONOPCO, INC. 2005-10-18 |
 VASELINE 77942194 4451609 Live/Registered |
Unilever Supply Chain, Inc. 2010-02-23 |
 VASELINE 77594219 4280087 Live/Registered |
Unilever Supply Chain, Inc. 2008-10-16 |
 VASELINE 76457132 2746932 Dead/Cancelled |
UNILEVER SUPPLY CHAIN, INC. 2002-10-09 |
 VASELINE 76274434 2532124 Live/Registered |
CONOPCO, INC. 2001-06-20 |
 VASELINE 74597738 2061852 Dead/Cancelled |
Conopco, Inc. 1994-11-10 |
 VASELINE 74546769 not registered Dead/Abandoned |
Conopco, Inc 1994-07-08 |
 VASELINE 71407423 0361324 Dead/Expired |
CHESEBROUGH MANUFACTURING COMPANY, CONSOLIDATED 1938-06-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.