ESCITALOPRAM OXALATE solution
Escitalopram Oxalate by
Drug Labeling and Warnings
Escitalopram Oxalate by is a Prescription medication manufactured, distributed, or labeled by Rising Pharma Holdings, Inc., Aurobindo Pharma Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ESCITALOPRAM ORAL SOLUTION safely and effectively. See full prescribing information for ESCITALOPRAM ORAL SOLUTION.
ESCITALOPRAM oral solution
Initial U.S. Approval: 2002WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
See full prescribing information for complete boxed warning.
Increased risk of suicidal thoughts and behavior in pediatric and young adult patients taking antidepressants. Closely monitor all antidepressant-treated patients for clinical worsening and emergence of suicidal thoughts and behaviors (5.1). Escitalopram oral solution is not approved for use in pediatric patients less than 7 years of age (8.4).
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
Indication and Population
Recommended Dosage
MDD in Adults (2.1)
Initial: 10 mg once daily
Recommended: 10 mg once daily Maximum: 20 mg once daily
MDD in Pediatric Patients 12 years and older (2.1)
Initial: 10 mg once daily
Recommended: 10 mg once daily
Maximum: 20 mg once daily
GAD in Adults (2.2)
Initial: 10 mg once daily
Recommended: 10 mg once daily
Maximum: 20 mg once daily
- No additional benefits were seen at 20 mg once daily (2.1)
- Administer once daily, morning or evening, with or without food (2.3)
- Elderly patients: recommended dosage is 10 mg once daily (2.4)
- Hepatic impairment: recommended dosage is 10 mg once daily (2.4, 8.6)
- When discontinuing escitalopram oral solution, reduce dose gradually whenever possible (2.5)
DOSAGE FORMS AND STRENGTHS
- Oral solution: 5 mg/5 mL
CONTRAINDICATIONS
- Do not use MAOIs intended to treat psychiatric disorders with escitalopram oral solution or within 14 days of stopping treatment with escitalopram oral solution. Do not use escitalopram oral solution within 14 days of stopping an MAOI intended to treat psychiatric disorders. In addition, do not start escitalopram oral solution in a patient who is being treated with linezolid or intravenous methylene blue (4)
- Concomitant use of pimozide (4)
- Known hypersensitivity to escitalopram or citalopram or any of the inactive ingredients (4)
WARNINGS AND PRECAUTIONS
- Serotonin Syndrome: Increased risk when co-administered with other serotonergic agents but also when taken alone. If it occurs, discontinue escitalopram oral solution and serotonergic agents and initiate supportive treatment (4, 5.2)
- Discontinuation syndrome: When discontinuing escitalopram oral solution, reduce dosage gradually whenever possible, and monitor for discontinuation symptoms (5.3)
- Seizures: Use with caution in patients with a history of seizure (5.4)
- Activation of Mania/Hypomania: Screen patients for bipolar disorder (5.5)
- Hyponatremia: Can occur in association with syndrome of inappropriate antidiuretic hormone secretion (5.6)
- Increased Risk of Bleeding: Concomitant use of nonsteroidal anti-inflammatory drugs, aspirin, other antiplatelet drugs, warfarin and other drugs that affect coagulation may increase risk (5.7)
- Interference with Cognitive and Motor Performance: Use caution when operating machinery (5.8)
- Angle Closure Glaucoma: Angle closure glaucoma has occurred in patients with untreated anatomically narrow angles treated with antidepressants (5.9)
- Use in Patients with Concomitant Illness: Use caution in patients with diseases or conditions that produce altered metabolism or hemodynamic responses (5.10)
- Sexual Dysfunction: Escitalopram oral solution may cause symptoms of sexual dysfunction (5.11)
ADVERSE REACTIONS
Most commonly observed adverse reactions (incidence ≥ 5% and at least twice the incidence of placebo patients) are: insomnia, ejaculation disorder (primarily ejaculatory delay), nausea, sweating increased, fatigue and somnolence, decreased libido, and anorgasmia (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Rising Pharma Holdings, Inc. at 1(844) 874-7464 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
- Pregnancy: SSRI use, particularly later in pregnancy, may increase the risk for persistent pulmonary hypertension and symptoms of poor adaptation (respiratory distress, temperature instability, feeding difficulties, hypotonia, tremor, irritability) in the neonate (8.1)
Additional pediatric use information is approved for AbbVie Inc.’s LEXAPRO (escitalopram) oral solution. However, due to AbbVie Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 5/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Major Depressive Disorder
2.2 Generalized Anxiety Disorder
2.3 Administration Information
2.4 Screen for Bipolar Disorder Prior to Starting Escitalopram Oral Solution
2.5 Recommended Dosage for Specific Populations
2.6 Discontinuation of Treatment with Escitalopram Oral Solution
2.7 Switching Patients to or from a Monoamine Oxidase Inhibitor (MAOI) Antidepressant
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Thoughts and Behaviors in Adolescents and Young Adults
5.2 Serotonin Syndrome
5.3 Discontinuation Syndrome
5.4 Seizures
5.5 Activation of Mania or Hypomania
5.6 Hyponatremia
5.7 Increased Risk of Bleeding
5.8 Interference with Cognitive and Motor Performance
5.9 Angle Closure Glaucoma
5.10 Use in Patients with Concomitant Illness
5.11 Sexual Dysfunction
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse and Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Major Depressive Disorder
14.2 Generalized Anxiety Disorder
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric and young adult patients in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening, and for emergence of suicidal thoughts and behaviors [see Warnings and Precautions (5.1)]. Escitalopram oral solution is not approved for use in pediatric patients less than 7 years of age [see Use in Specific Populations (8.4)].
-
1 INDICATIONS AND USAGE
Escitalopram oral solution is indicated for the treatment of:
- major depressive disorder (MDD) in adults and pediatric patients 12 years of age and older.
- generalized anxiety disorder (GAD) in adults.
Additional pediatric use information is approved for AbbVie Inc.’s LEXAPRO (escitalopram) oral solution. However, due to AbbVie Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
-
2 DOSAGE AND ADMINISTRATION
2.1 Major Depressive Disorder
Adults
The recommended dosage of escitalopram oral solution in adults is 10 mg once daily. A fixed-dose trial of escitalopram oral solution demonstrated the effectiveness of both 10 mg and 20 mg of escitalopram oral solution, but failed to demonstrate a greater benefit of 20 mg over 10 mg [see Clinical Studies (14.1)]. Depending on clinical response and tolerability, dosage may be increased to the maximum recommended dosage of 20 mg once daily at an interval of no less than 1 week.
Pediatric Patients 12 years of age and older
The recommended dosage of escitalopram oral solution in pediatric patients 12 years of age and older is 10 mg once daily. Depending on clinical response and tolerability, dosage may be increased to the maximum recommended dosage of 20 mg once daily at an interval of no less than 3 weeks.2.2 Generalized Anxiety Disorder
Adults
The recommended starting dosage of escitalopram oral solution in adults is 10 mg once daily. Depending on clinical response and tolerability, dosage may be increased to the maximum recommended dosage of 20 mg once daily at an interval of no less than 1 week.
Additional pediatric use information is approved for AbbVie Inc.’s LEXAPRO (escitalopram) oral solution. However, due to AbbVie Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
2.3 Administration Information
Administer escitalopram oral solution orally once daily, in the morning or evening, with or without food.
2.4 Screen for Bipolar Disorder Prior to Starting Escitalopram Oral Solution
Prior to initiating treatment with escitalopram oral solution or another antidepressant, screen patients for a personal family history of bipolar disorder, mania, or hypomania [see Warnings and Precautions (5.5)].
2.5 Recommended Dosage for Specific Populations
The recommended dosage for most elderly patients and patients with hepatic impairment is 10 mg once daily [see Use in Specific Populations (8.5, 8.6)].
The recommended dosage for escitalopram oral solution in adults with a creatinine clearance less than 20 mL/minute has not been determined. No dosage adjustment is necessary for patients with mild or moderate renal impairment [see Use in Specific Populations (8.7)].
2.6 Discontinuation of Treatment with Escitalopram Oral Solution
Symptoms associated with discontinuation of escitalopram oral solution and other SSRIs and SNRIs have been reported [see Warnings and Precautions (5.3)]. Patients should be monitored for these symptoms when discontinuing treatment. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose but at a more gradual rate.
2.7 Switching Patients to or from a Monoamine Oxidase Inhibitor (MAOI) Antidepressant
At least 14 days should elapse between discontinuation of an MAOI intended to treat psychiatric disorders and initiation of therapy with escitalopram oral solution. Conversely, at least 14 days should be allowed after stopping escitalopram oral solution before starting an MAOI intended to treat psychiatric disorders [see Contraindications (4)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Escitalopram oral solution is contraindicated in patients:
- taking MAOIs with escitalopram oral solution or within 14 days of stopping treatment with escitalopram oral solution because of an increased risk of serotonin syndrome. The use of escitalopram oral solution within 14 days of stopping an MAOI intended to treat psychiatric disorders is also contraindicated [see Dosage and Administration (2.7) and Warnings and Precautions (5.2)]. Starting escitalopram oral solution in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue is also contraindicated because of an increased risk of serotonin syndrome [see Dosage and Administration (2.6) and Warnings and Precautions (5.2)].
- taking pimozide [see Drug Interactions (7)].
- with a hypersensitivity to escitalopram or citalopram or any of the inactive ingredients in escitalopram oral solution.
-
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Thoughts and Behaviors in Adolescents and Young Adults
In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other antidepressant classes) that included approximately 77,000 adult patients and 4,500 pediatric patients, the incidence of suicidal thoughts and behaviors in the antidepressant-treated patients age 24 years and younger was greater than in placebo-treated patients. There was considerable variation in risk of suicidal thoughts and behaviors among drugs, but there was an increased risk identified in young patients for most drugs studied. There were differences in absolute risk of suicidal thoughts and behaviors across the different indications, with the highest incidence in patients with MDD. The drug-placebo differences in the number of cases of suicidal thoughts and behaviors per 1000 patients treated are provided in Table 1.
Table 1: Risk Differences of the Number of Patients of Suicidal Thoughts and Behaviors in the Pooled Placebo-Controlled Trials of Antidepressants in Pediatric and Adult Patients
Age Range
Drug-Placebo Difference in Number of
Patients of Suicidal Thoughts and Behaviors per 1000 Patients Treated
Increases Compared to Placebo
<18 years old
14 additional patients
18 to 24 years old
5 additional patients
Decreases Compared to Placebo
25 to 64 years old
1 fewer patient
≥65 years old
6 fewer patients
It is unknown whether the risk of suicidal thoughts and behaviors in children, adolescents, and young adults extends to longer-term use, i.e., beyond four months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with MDD that antidepressants delay the recurrence of depression and that depression itself is a risk factor for suicidal thoughts and behaviors.
Monitor all antidepressant-treated patients for any indication for clinical worsening and emergence of suicidal thoughts and behaviors, especially during the initial few months of drug therapy, and at times of dosage changes. Counsel family members or caregivers of patients to monitor for changes in behavior and to alert the healthcare provider. Consider changing the therapeutic regimen, including possibly discontinuing escitalopram oral solution, in patients whose depression is persistently worse, or who are experiencing emergent suicidal thoughts or behaviors.
5.2 Serotonin Syndrome
SSRIs, including escitalopram oral solution, can precipitate serotonin syndrome, a potentially life-threatening condition. The risk is increased with concomitant use of other serotonergic drugs (including triptans, tricyclic antidepressants, fentanyl, meperidine, methadone, lithium, tramadol, tryptophan, buspirone, amphetamines, and St. John’s Wort) and with drugs that impair metabolism of serotonin, i.e., MAOIs [see Contraindications (4) and Drug Interactions (7)].
Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination) seizures, and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
The concomitant use of escitalopram oral solution with MAOIs is contraindicated. In addition, do not initiate escitalopram oral solution in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue. No reports involved the administration of methylene blue by other routes (such as oral tablets or local tissue injection). If it is necessary to initiate treatment with an MAOI such as linezolid or intravenous methylene blue in a patient taking escitalopram oral solution, discontinue escitalopram oral solution before initiating treatment with the MAOI [see Contraindications (4) and Dosage and Administration (2.7)].
Monitor all patients taking escitalopram oral solution for the emergence of serotonin syndrome. Discontinue treatment with escitalopram oral solution and any concomitant serotonergic agents immediately if the above symptoms occur, and initiate supportive symptomatic treatment. If concomitant use of escitalopram oral solution with other serotonergic drugs is clinically warranted, inform patients of the increased risk for serotonin syndrome and monitor for symptoms.
5.3 Discontinuation Syndrome
During marketing of escitalopram oral solution and other SSRIs, there have been spontaneous reports of adverse reactions occurring upon discontinuation of these drugs, particularly when abrupt, including the following: dysphoric mood, irritability, agitation, dizziness, sensory disturbances (e.g., paresthesias such as electric shock sensations), anxiety, confusion, headache, lethargy, emotional lability, insomnia, and hypomania. While these events are generally self-limiting, there have been reports of serious discontinuation symptoms.
Monitor for these symptoms when discontinuing treatment with escitalopram oral solution. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose but at a more gradual rate [see Dosage and Administration (2.6)].5.4 Seizures
Although anticonvulsant effects of racemic citalopram have been observed in animal studies, escitalopram oral solution has not been systematically evaluated in patients with a seizure disorder. These patients were excluded from clinical studies during the product's premarketing testing. In clinical trials of escitalopram oral solution, cases of convulsion have been reported in association with escitalopram oral solution treatment. Like other drugs effective in the treatment of major depressive disorder, escitalopram oral solution should be introduced with care in patients with a history of seizure disorder.
5.5 Activation of Mania or Hypomania
In patients with bipolar disorder, treating a depressive episode with escitalopram oral solution or another antidepressant may precipitate a mixed/manic episode. In placebo-controlled trials of escitalopram oral solution in major depressive disorder, activation of mania/hypomania was reported in one (0.1%) of 715 patients treated with escitalopram oral solution and in none of the 592 patients treated with placebo. One additional case of hypomania has been reported in association with escitalopram oral solution treatment. Activation of mania/hypomania has also been reported in a small proportion of patients with major affective disorders treated with racemic citalopram and other marketed drugs effective in the treatment of major depressive disorder. Prior to initiating treatment with escitalopram oral solution, screen patients for any personal or family history of bipolar disorder, mania, or hypomania [see Dosage and Administration (2.4)].
5.6 Hyponatremia
Hyponatremia may occur as a result of treatment with SSRIs, including escitalopram oral solution. In many cases, this hyponatremia appears to be the result of the syndrome of inappropriate antidiuretic hormone secretion (SIADH), and was reversible when escitalopram oral solution was discontinued. Cases with serum sodium lower than 110 mmol/L have been reported. Elderly patients may be at greater risk of developing hyponatremia with SSRIs and SNRIs. Also, patients taking diuretics or who are otherwise volume depleted may be at greater risk [see Use in Specific Populations (8.5)]. Consider discontinuation of escitalopram oral solution in patients with symptomatic hyponatremia and appropriate medical intervention should be instituted.
Signs and symptoms of hyponatremia include headache, difficulty concentrating, memory impairment, confusion, weakness, and unsteadiness, which may lead to falls. Signs and symptoms associated with more severe and/or acute cases have included hallucination, syncope, seizure, coma, respiratory arrest, and death.5.7 Increased Risk of Bleeding
Drugs that interfere with serotonin reuptake inhibition, including escitalopram oral solution, increase the risk of bleeding events. Concomitant use of aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), other antiplatelet drugs, warfarin, and other anticoagulants may add to the risk. Case reports and epidemiological studies (case-control and cohort design) have demonstrated an association between use of drugs that interfere with serotonin reuptake and the occurrence of gastrointestinal bleeding. Based on data from the published observational studies, exposure to SSRIs, particularly in the month before delivery, has been associated with a less than 2-fold increase in the risk of postpartum hemorrhage [see Use in Specific Populations (8.1)]. Bleeding events related to drugs that interfere with serotonin reuptake have ranged from ecchymoses, hematomas, epistaxis, and petechiae to life-threatening hemorrhages.
Inform patients about the increased risk of bleeding associated with the concomitant use of escitalopram oral solution and antiplatelet agents or anticoagulants. For patients taking warfarin, carefully monitor the international normalized ratio [see Drug Interactions (7)].
5.8 Interference with Cognitive and Motor Performance
In a study in normal volunteers, escitalopram oral solution 10 mg daily did not produce impairment of intellectual function or psychomotor performance. Because any psychoactive drug may impair judgment, thinking, or motor skills, however, patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that escitalopram oral solution therapy does not affect their ability to engage in such activities.
5.9 Angle Closure Glaucoma
The pupillary dilation that occurs following use of many antidepressant drugs, including escitalopram oral solution may trigger an angle closure attack in a patient with anatomically narrow angles who does not have a patent iridectomy.
5.10 Use in Patients with Concomitant Illness
Clinical experience with escitalopram oral solution in patients with certain concomitant systemic illnesses is limited. Caution is advisable in using escitalopram oral solution in patients with diseases or conditions that produce altered metabolism or hemodynamic responses.
Escitalopram oral solution has not been systematically evaluated in patients with a recent history of myocardial infarction or unstable heart disease. Patients with these diagnoses were generally excluded from clinical studies during the product's premarketing testing.
In subjects with hepatic impairment, clearance of racemic citalopram was decreased and plasma concentrations were increased. The recommended dose of escitalopram oral solution in hepatically impaired patients is 10 mg daily [see Dosage and Administration (2.5) and Use in Specific Populations (8.6)].
Because escitalopram is extensively metabolized, excretion of unchanged drug in urine is a minor route of elimination. Until adequate numbers of patients with severe renal impairment have been evaluated during chronic treatment with escitalopram oral solution, however, it should be used with caution in such patients [see Dosage and Administration (2.5) and Use in Specific Populations (8.7)].
5.11 Sexual Dysfunction
Use of SSRIs, including escitalopram oral solution, may cause symptoms of sexual dysfunction [see Adverse Reactions (6.1)]. In male patients, SSRI use may result in ejaculatory delay or failure, decreased libido, and erectile dysfunction. In female patients, SSRI use may result in decreased libido and delayed or absent orgasm.
It is important for prescribers to inquire about sexual function prior to initiation of escitalopram oral solution and to inquire specifically about changes in sexual function during treatment, because sexual function may not be spontaneously reported. When evaluating changes in sexual function, obtaining a detailed history (including timing of symptom onset) is important because sexual symptoms may have other causes, including the underlying psychiatric disorder. Discuss potential management strategies to support patients in making informed decisions about treatment.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Suicidal thoughts and behaviors in adolescents and young adults [see Warnings and Precautions (5.1)]
- Serotonin syndrome [see Warnings and Precautions (5.2)]
- Discontinuation syndrome [see Warnings and Precautions (5.3)]
- Seizures [see Warnings and Precautions (5.4)]
- Activation of mania or hypomania [see Warnings and Precautions (5.5)]
- Hyponatremia [see Warnings and Precautions (5.6)]
- Increased Risk of Bleeding [see Warnings and Precautions (5.7)]
- Interference with Cognitive and Motor Performance [see Warnings and Precautions (5.8)]
- Angle-closure glaucoma [see Warnings and Precautions (5.9)]
- Use in Patients with Concomitant Illness [see Warnings and Precautions (5.10)]
- Sexual Dysfunction [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Clinical Trial Data Sources
Adults
Adverse reactions information for escitalopram oral solution was collected from 715 patients with major depressive disorder who were exposed to escitalopram and from 592 patients who were exposed to placebo in double-blind, placebo-controlled trials. An additional 284 patients with major depressive disorder were newly exposed to escitalopram in open-label trials. The adverse reaction information for escitalopram oral solution in patients with GAD was collected from 429 patients exposed to escitalopram and from 427 patients exposed to placebo in double-blind, placebo-controlled trials.
Adverse reactions during exposure were obtained primarily by general inquiry and recorded by clinical investigators using terminology of their own choosing. Consequently, it is not possible to provide a meaningful estimate of the proportion of individuals experiencing adverse reactions without first grouping similar types of reactions into a smaller number of standardized event categories. In the tables and tabulations that follow, standard World Health Organization (WHO) terminology has been used to classify reported adverse reactions.
The stated frequencies of adverse reactions represent the proportion of individuals who experienced, at least once, a treatment emergent adverse event of the type listed. An event was considered treatment-emergent if it occurred for the first time or worsened while receiving therapy following baseline evaluation.
Pediatric Patients
Adverse reaction information for pediatric patients was collected in double-blind placebo-controlled studies in 576 pediatric patients 6 to 17 years of age, (286 escitalopram oral solution, 290 placebo) with major depressive disorder.
The safety and effectiveness of escitalopram oral solution have not been established in pediatric patients less than 12 years of age with MDD or less than 7 years of age with GAD.
Adverse Reactions Associated with Discontinuation of Treatment
Major Depressive Disorder
Adults
Among the 715 depressed patients who received escitalopram oral solution in placebo-controlled trials, 6% discontinued treatment due to an adverse event, as compared to 2% of 592 patients receiving placebo. In two fixed-dose studies, the rate of discontinuation for adverse reactions in patients receiving 10 mg/day escitalopram oral solution was not significantly different from the rate of discontinuation for adverse reactions in patients receiving placebo. The rate of discontinuation for adverse reactions in patients assigned to a fixed dose of 20 mg/day escitalopram oral solution was 10%, which was significantly different from the rate of discontinuation for adverse reactions in patients receiving 10 mg/day escitalopram oral solution (4%) and placebo (3%). Adverse reactions that were associated with the discontinuation of at least 1% of patients treated with escitalopram oral solution, and for which the rate was at least twice that of placebo, were nausea (2%) and ejaculation disorder (2% of male patients).
Pediatric Patients
Adverse reactions in pediatric patients 6 to 17 years of age were associated with discontinuation of 3.5% of 286 patients receiving escitalopram oral solution and 1% of 290 patients receiving placebo. The most common adverse reaction (incidence at least 1% for escitalopram oral solution and greater than placebo) associated with discontinuation was insomnia (1% escitalopram oral solution, 0% placebo).
The safety and effectiveness of escitalopram oral solution have not been established in pediatric patients less than 12 years of age with MDD.
Generalized Anxiety Disorder
Adults
Among the 429 GAD patients who received escitalopram oral solution 10 to 20 mg/day in placebo-controlled trials, 8% discontinued treatment due to an adverse event, as compared to 4% of 427 patients receiving placebo. Adverse reactions that were associated with the discontinuation of at least 1% of patients treated with escitalopram oral solution, and for which the rate was at least twice the placebo rate, were nausea (2%), insomnia (1%), and fatigue (1%).
Incidence of Adverse Reactions in Placebo-Controlled Clinical Trials
Major Depressive Disorder
Adults
The most commonly observed adverse reactions in escitalopram oral solution patients (incidence of approximately 5% or greater and approximately twice the incidence in placebo patients) were insomnia, ejaculation disorder (primarily ejaculatory delay), nausea, sweating increased, fatigue, and somnolence.
Table 2 enumerates the incidence, rounded to the nearest percent, of adverse reactions that occurred among 715 depressed patients who received escitalopram oral solution at doses ranging from 10 to 20 mg/day in placebo-controlled trials. Reactions included are those occurring in 2% or more of patients treated with escitalopram oral solution and for which the incidence in patients treated with escitalopram oral solution was greater than the incidence in placebo-treated patients.
TABLE 2
Adverse Reactions observed with a frequency of ≥ 2% and greater than placebo for Major Depressive Disorder (Adults)
Adverse Reaction
Escitalopram oral solution
Placebo
(N=715)
%
(N=592)
%
Autonomic Nervous System Disorders
Dry Mouth
6%
5%
Sweating Increased
5%
2%
Central & Peripheral Nervous System Disorders
Dizziness
5%
3%
Gastrointestinal Disorders
Nausea
15%
7%
Diarrhea
8%
5%
Constipation
3%
1%
Indigestion
3%
1%
Abdominal Pain
2%
1%
General
Influenza-like Symptoms
5%
4%
Fatigue
5%
2%
Psychiatric Disorders
Insomnia
9%
4%
Somnolence
6%
2%
Appetite Decreased
3%
1%
Libido Decreased
3%
1%
Respiratory System Disorders
Rhinitis
5%
4%
Sinusitis
3%
2%
Urogenital
Ejaculation Disorder1,2
9%
<1%
Impotence2
3%
<1%
Anorgasmia3
2%
<1%
1Primarily ejaculatory delay.
2Denominator used was for males only (N=225 escitalopram oral solution; N=188 placebo).
3Denominator used was for females only (N=490 escitalopram oral solution; N=404 placebo).
Pediatric Patients
The overall profile of adverse reactions in pediatric patients 6 to 17 years in major depressive disorder was generally similar to that seen in adult studies, as shown in Table 2. However, the following adverse reactions (excluding those which appear in Table 2 and those for which the coded terms were uninformative or misleading) were reported at an incidence of at least 2% for escitalopram oral solution and greater than placebo: back pain, urinary tract infection, vomiting, and nasal congestion.
The safety and effectiveness of escitalopram oral solution have not been established in pediatric patients less than 12 years of age with MDD.
Generalized Anxiety Disorder
Adults
The most commonly observed adverse reactions in escitalopram oral solution patients (incidence of approximately 5% or greater and approximately twice the incidence in placebo patients) were nausea, ejaculation disorder (primarily ejaculatory delay), insomnia, fatigue, decreased libido, and anorgasmia.
Table 3 enumerates the incidence, rounded to the nearest percent of treatment-emergent adverse reactions that occurred among 429 GAD patients who received escitalopram oral solution 10 to 20 mg/day in placebo-controlled trials. Reactions included are those occurring in 2% or more of patients treated with escitalopram oral solution and for which the incidence in patients treated with escitalopram oral solution was greater than the incidence in placebo-treated patients.
TABLE 3
Adverse Reactions Observed with a Frequency of ≥ 2% and> placebo for Generalized Anxiety Disorder (Adults)
Adverse Reactions
Escitalopram oral solution
Placebo
(N=429)
%
(N=427)
%
Autonomic Nervous System Disorders
Dry Mouth
9%
5%
Sweating Increased
4%
1%
Central & Peripheral Nervous System Disorders
Headache
24%
17%
Paresthesia
2%
1%
Gastrointestinal Disorders
Nausea
18%
8%
Diarrhea
8%
6%
Constipation
5%
4%
Indigestion
3%
2%
Vomiting
3%
1%
Abdominal Pain
2%
1%
Flatulence
2%
1%
Toothache
2%
0%
General
Fatigue
8%
2%
Influenza-like Symptoms
5%
4%
Musculoskeletal System Disorder
Neck/Shoulder Pain
3%
1%
Psychiatric Disorders
Somnolence
13%
7%
Insomnia
12%
6%
Libido Decreased
7%
2%
Dreaming Abnormal
3%
2%
Appetite Decreased
3%
1%
Lethargy
3%
1%
Respiratory System Disorders
Yawning
2%
1%
Urogenital
Ejaculation Disorder1,2
14%
2%
Anorgasmia3
6%
<1%
Menstrual Disorder
2%
1%
1Primarily ejaculatory delay.
2Denominator used was for males only (N=182 escitalopram oral solution; N=195 placebo).
3Denominator used was for females only (N=247 escitalopram oral solution; N=232 placebo).
Dose Dependency of Adverse Reactions
The potential dose dependency of common adverse reactions (defined as an incidence rate of ≥5% in either the 10 mg or 20 mg escitalopram oral solution groups) was examined on the basis of the combined incidence of adverse reactions in two fixed-dose trials. The overall incidence rates of adverse reactions in 10 mg escitalopram oral solution-treated patients (66%) was similar to that of the placebo-treated patients (61%), while the incidence rate in 20 mg/day escitalopram oral solution-treated patients was greater (86%). Table 4 shows common adverse reactions that occurred in the 20 mg/day escitalopram oral solution group with an incidence that was approximately twice that of the 10 mg/day escitalopram oral solution group and approximately twice that of the placebo group.
TABLE 4
Incidence of Common Adverse Reactions in Patients with Major Depressive Disorder
Adverse Reaction
Placebo
10 mg/day
20 mg/day
(N=311)
Escitalopram oral solution
Escitalopram oral solution
(N=310)
(N=125)
Insomnia
4%
7%
14%
Diarrhea
5%
6%
14%
Dry Mouth
3%
4%
9%
Somnolence
1%
4%
9%
Dizziness
2%
4%
7%
Sweating Increased
<1%
3%
8%
Constipation
1%
3%
6%
Fatigue
2%
2%
6%
Indigestion
1%
2%
6%
Male and Female Sexual Dysfunction with SSRIs
Although changes in sexual desire, sexual performance, and sexual satisfaction often occur as manifestations of a psychiatric disorder, they may also be a consequence of pharmacologic treatment. In particular, some evidence suggests that SSRIs can cause such untoward sexual experiences.
Reliable estimates of the incidence and severity of untoward experiences involving sexual desire, performance, and satisfaction are difficult to obtain, however, in part because patients and physicians may be reluctant to discuss them. Accordingly, estimates of the incidence of untoward sexual experience and performance cited in product labeling are likely to underestimate their actual incidence.
TABLE 5
Incidence of Sexual Side Effects in Placebo-Controlled Clinical Trials
Adverse Event
Escitalopram oral solution
Placebo
In Males Only
(N=407)
(N=383)
Ejaculation Disorder
(primarily ejaculatory delay)
12%
1%
Libido Decreased
6%
2%
Impotence
2%
<1%
In Females Only
(N=737)
(N=636)
Libido Decreased
3%
1%
Anorgasmia
3%
<1%
There are no adequately designed studies examining sexual dysfunction with escitalopram treatment.
Priapism has been reported with all SSRIs.
While it is difficult to know the precise risk of sexual dysfunction associated with the use of SSRIs, physicians should routinely inquire about such possible side effects.
Vital Sign Changes
Escitalopram oral solution and placebo groups were compared with respect to (1) mean change from baseline in vital signs (pulse, systolic blood pressure, and diastolic blood pressure) and (2) the incidence of patients meeting criteria for potentially clinically significant changes from baseline in these variables. These analyses did not reveal any clinically important changes in vital signs associated with escitalopram oral solution treatment. In addition, a comparison of supine and standing vital sign measures in subjects receiving escitalopram oral solution indicated that escitalopram oral solution treatment is not associated with orthostatic changes.
Weight Changes
Patients treated with escitalopram oral solution in controlled trials did not differ from placebo-treated patients with regard to clinically important change in body weight.
Laboratory Changes
Escitalopram oral solution and placebo groups were compared with respect to (1) mean change from baseline in various serum chemistry, hematology, and urinalysis variables, and (2) the incidence of patients meeting criteria for potentially clinically significant changes from baseline in these variables. These analyses revealed no clinically important changes in laboratory test parameters associated with escitalopram oral solution treatment.
ECG Changes
Electrocardiograms from escitalopram oral solution (N=625) and placebo (N=527) groups were compared with respect to outliers defined as subjects with QTc changes over 60 msec from baseline or absolute values over 500 msec post-dose, and subjects with heart rate increases to over 100 bpm or decreases to less than 50 bpm with a 25% change from baseline (tachycardic or bradycardic outliers, respectively). None of the patients in the escitalopram oral solution group had a QTcF interval >500 msec or a prolongation >60 msec compared to 0.2% of patients in the placebo group. The incidence of tachycardic outliers was 0.2% in the escitalopram oral solution and the placebo group. The incidence of bradycardic outliers was 0.5% in the escitalopram oral solution group and 0.2% in the placebo group.
QTcF interval was evaluated in a randomized, placebo and active (moxifloxacin 400 mg) controlled cross-over, escalating multiple dose study in 113 healthy subjects. The maximum mean (95% upper confidence bound) difference from placebo arm were 4.5 (6.4) and 10.7 (12.7) msec for 10 mg and supratherapeutic 30 mg escitalopram given once daily, respectively. Based on the established exposure-response relationship, the predicted QTcF change from placebo arm (95% confidence interval) under the Cmax for the dose of 20 mg is 6.6 (7.9) msec. Escitalopram 30 mg given once daily resulted in mean Cmax of 1.7-fold higher than the mean Cmax for the maximum recommended therapeutic dose at steady state (20 mg). The exposure under supratherapeutic 30 mg dose is similar to the steady state concentrations expected in CYP2C19 poor metabolizers following a therapeutic dose of 20 mg.
Other Reactions Observed During the Premarketing Evaluation of Escitalopram Oral Solution
Following is a list of treatment-emergent adverse reactions, as defined in the introduction to the ADVERSE REACTIONS section, reported by the 1428 patients treated with escitalopram oral solution for periods of up to one year in double-blind or open-label clinical trials during its premarketing evaluation. The listing does not include those reactions already listed in Tables 2 & 3, those reactions for which a drug cause was remote and at a rate less than 1% or lower than placebo, those reactions which were so general as to be uninformative, and those reactions reported only once which did not have a substantial probability of being acutely life threatening. Reactions are categorized by body system. Reactions of major clinical importance are described in the Warnings and Precautions section (5).
Cardiovascular: hypertension, palpitation.
Central and Peripheral Nervous System Disorders: light-headed feeling, migraine.
Gastrointestinal Disorders: abdominal cramp, heartburn, gastroenteritis.
General: allergy, chest pain, fever, hot flushes, pain in limb.
Metabolic and Nutritional Disorders: increased weight.
Musculoskeletal System Disorders: arthralgia, myalgia jaw stiffness.
Psychiatric Disorders: appetite increased, concentration impaired, irritability.
Reproductive Disorders/Female: menstrual cramps, menstrual disorder.
Respiratory System Disorders: bronchitis, coughing, nasal congestion, sinus congestion, sinus headache.
Skin and Appendages Disorders: rash.
Special Senses: vision blurred, tinnitus.
Urinary System Disorders: urinary frequency, urinary tract infection.
Additional pediatric use information is approved for AbbVie Inc.’s LEXAPRO (escitalopram) oral solution. However, due to AbbVie Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
6.2 Post-Marketing Experience
Adverse Reactions Reported Subsequent to the Marketing of Escitalopram
The following adverse reactions have been identified during post-approval use of escitalopram oral solution. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: anemia, agranulocytis, aplastic anemia, hemolytic anemia, idiopathic thrombocytopenia purpura, leukopenia, thrombocytopenia.
Cardiac Disorders: atrial fibrillation, bradycardia, cardiac failure, myocardial infarction, tachycardia, torsade de pointes, ventricular arrhythmia, ventricular tachycardia.
Ear and Labyrinth Disorders: vertigo.
Endocrine Disorders: diabetes mellitus, hyperprolactinemia, SIADH.
Eye Disorders: angle closure glaucoma, diplopia, mydriasis, visual disturbance.
Gastrointestinal Disorder: dysphagia, gastrointestinal hemorrhage, gastroesophageal reflux, pancreatitis, rectal hemorrhage.
General Disorders and Administration Site Conditions: abnormal gait, asthenia, edema, fall, feeling abnormal, malaise.
Hepatobiliary Disorders: fulminant hepatitis, hepatic failure, hepatic necrosis, hepatitis.
Immune System Disorders: allergic reaction, anaphylaxis.
Investigations: bilirubin increased, decreased weight, electrocardiogram QT prolongation, hepatic enzymes increased, hypercholesterolemia, INR increased, prothrombin decreased.
Metabolism and Nutrition Disorders: hyperglycemia, hypoglycemia, hypokalemia, hyponatremia.
Musculoskeletal and Connective Tissue Disorders: muscle cramp, muscle stiffness, muscle weakness, rhabdomyolysis.
Nervous System Disorders: akathisia, amnesia, ataxia, choreoathetosis, cerebrovascular accident, dysarthria, dyskinesia, dystonia, extrapyramidal disorders, grand mal seizures (or convulsions), hypoaesthesia, myoclonus, nystagmus, Parkinsonism, restless legs, seizures, syncope, tardive dyskinesia, tremor.
Pregnancy, Puerperium and Perinatal Conditions: spontaneous abortion.
Psychiatric Disorders: acute psychosis, aggression, agitation, anger, anxiety, apathy, completed suicide, confusion, depersonalization, depression aggravated, delirium, delusion, disorientation, feeling unreal, hallucinations (visual and auditory), mood swings, nervousness, nightmare, panic reaction, paranoia, restlessness, self-harm or thoughts of self-harm, suicide attempt, suicidal ideation, suicidal tendency.
Renal and Urinary Disorders: acute renal failure, dysuria, urinary retention.
Reproductive System and Breast Disorders: menorrhagia, priapism.
Respiratory, Thoracic and Mediastinal Disorders: anosmia, dyspnea, epistaxis, pulmonary embolism, hyposmia, pulmonary hypertension of the newborn.
Skin and Subcutaneous Tissue Disorders: alopecia, angioedema, dermatitis, drug reaction with eosinophilia and systemic symptoms (DRESS), ecchymosis, erythema multiforme, photosensitivity reaction, Stevens Johnson Syndrome, toxic epidermal necrolysis, urticaria.
Vascular Disorders: deep vein thrombosis, flushing, hypertensive crisis, hypotension, orthostatic hypotension, phlebitis, thrombosis.
-
7 DRUG INTERACTIONS
Table 6 presents clinically important drug interactions with escitalopram oral solution.
TABLE 6 Clinically Important Drug Interactions with Escitalopram Oral Solution Monoamine Oxidase Inhibitors (MAOIs)
Clinical
Impact:
Concomitant use of SSRIs, including escitalopram oral solution, and MAOIs increases the risk of serotonin syndrome.
Intervention:
Escitalopram oral solution is contraindicated in patients taking MAOIs, including MAOIs such as linezolid or intravenous methylene blue [see Dosage and Administration (2.7), Contraindications (4), and Warnings and Precautions (5.2)].
Pimozide
Clinical
Impact:
Concomitant use of racemic citalopram with pimozide increases plasma concentrations of pimozide, a drug with a narrow therapeutic index, and may increase the risk of QT prolongation and/or ventricular arrhythmias compared to use of racemic citalopram alone [see Clinical Pharmacology (12.3)].
Intervention:
Escitalopram oral solution is contraindicated in patients taking pimozide [see Contraindications (4)].
Other Serotonergic Drugs
Clinical
Impact:
Concomitant use of escitalopram oral solution and other serotonergic drugs (including other SSRIs, SNRIs, triptans, tricyclic antidepressants, opioids, lithium, buspirone, amphetamines, tryptophan, and St. John's Wort) increases the risk of serotonin syndrome.
Intervention:
Monitor patients for signs and symptoms of serotonin syndrome, particularly during escitalopram oral solution initiation and dosage increases. If serotonin syndrome occurs, consider discontinuation of escitalopram oral solution and/or concomitant serotonergic drugs [see Warning and Precautions (5.2)].
Drugs That Interfere With Hemostasis (NSAIDs, Aspirin, Warfarin, etc.)
Clinical
Impact:
Concomitant use of escitalopram oral solution and an antiplatelet or anticoagulant may potentiate the risk of bleeding.
Intervention:
Inform patients of the increased risk of bleeding associated with the concomitant use of escitalopram oral solution and antiplatelet agents and anticoagulants. For patients taking warfarin, carefully monitor the international normalized ratio [see Warning and Precautions (5.7)].
Sumatriptan
Clinical
Impact:
There have been postmarketing reports describing patients with weakness, hyperreflexia, and incoordination following the use of an SSRI and sumatriptan.
Intervention:
If concomitant treatment with sumatriptan and an SSRI is clinically warranted, appropriate observation of the patient is advised [see Warning and Precautions (5.2)].
Carbamazepine
Clinical
Impact:
Combined administration of racemic citalopram (40 mg/day for 14 days) and carbamazepine (titrated to 400 mg/day for 35 days) did not significantly affect the pharmacokinetics of carbamazepine, a CYP3A4 substrate.
Intervention:
Although trough citalopram plasma levels were unaffected, given the enzyme-inducing properties of carbamazepine, the possibility that carbamazepine might increase the clearance of escitalopram should be considered if the two drugs are coadministered.
Drugs Metabolized by CYP2D6
Clinical
Impact:
Coadministration of escitalopram (20 mg/day for 21 days) with the tricyclic antidepressant desipramine (single dose of 50 mg), a substrate for CYP2D6, resulted in a 40% increase in Cmax and a 100% increase in AUC of desipramine.
Intervention:
The clinical significance of this finding is unknown. Exercise caution during coadministration of escitalopram and drugs metabolized by CYP2D6.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antidepressants during pregnancy. Healthcare providers are encouraged to advise patients to register by calling the National Pregnancy Registry for Antidepressants at 1-844-405-6185 or visiting online at https://womensmentalhealth.org/research/pregnancyregistry/antidepressants.
Risk Summary
Based on data from published observational studies, exposure to SSRIs, particularly in the month before delivery, has been associated with a less than 2-fold increase in the risk of postpartum hemorrhage [see Warnings and Precautions (5.7) and Clinical Considerations].
Available data from published epidemiologic studies and postmarketing reports have not established an increased risk of major birth defects or miscarriage. There are risks of persistent pulmonary hypertension of the newborn (PPHN) (see Data) and poor neonatal adaptation (see Clinical Considerations) with exposure to selective serotonin reuptake inhibitors (SSRIs), including escitalopram oral solution, during pregnancy. There are risks associated with untreated depression in pregnancy (see Clinical Considerations).
In animal reproduction studies, both escitalopram and racemic citalopram have been shown to have adverse effects on embryo/fetal and postnatal development, including fetal structural abnormalities, when administered at doses greater than human therapeutic doses (see Data).The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in the clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-associated maternal risk and/or embryo/fetal risk
Women who discontinue antidepressants are more likely to experience a relapse of major depression than women who continue antidepressants. This finding is from a prospective longitudinal study of 201 pregnant women with a history of major depression, who were euthymic and taking antidepressants at the beginning of pregnancy. Consider the risk of untreated depression when discontinuing or changing treatment with antidepressant medication during pregnancy and postpartum.
Maternal Adverse Reactions
Use of escitalopram oral solution in the month before delivery may be associated with an increased risk of postpartum hemorrhage [see Warnings and Precautions (5.7)].
Fetal/Neonatal adverse reactions
Neonates exposed to SSRIs or SNRIs, including escitalopram oral solution, late in third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding. Such complications can arise immediately upon delivery. Reported clinical findings have included respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremor, jitteriness, irritability, and constant crying. These features are consistent with either a direct toxic effect of SSRIs and SNRIs or, possibly, a drug discontinuation syndrome. It should be noted that, in some cases, the clinical picture is consistent with serotonin syndrome [see Warnings and Precautions (5.2)].
Data
Human Data
Exposure to SSRIs, particularly later in pregnancy, may increase the risk for PPHN. PPHN occurs in 1 to 2 per 1000 live births in the general populations and is associated with substantial neonatal morbidity and mortality.
Animal Data
In a rat embryo/fetal development study, oral administration of escitalopram (56, 112, or 150 mg/kg/day) to pregnant animals during the period of organogenesis resulted in decreased fetal body weight and associated delays in ossification at the two higher doses [approximately ≥ 55 times the maximum recommended human dose (MRHD) of 20 mg/day on a mg/m2 basis]. Maternal toxicity (clinical signs and decreased body weight gain and food consumption), mild at 56 mg/kg/day, was present at all dose levels. The developmental no-effect dose of 56 mg/kg/day is approximately 27 times the MRHD of 20 mg on a mg/m2 basis. No malformations were observed at any of the doses tested (as high as 73 times the MRHD on a mg/m2 basis).
When female rats were treated with escitalopram (6, 12, 24, or 48 mg/kg/day) during pregnancy and through weaning, slightly increased offspring mortality and growth retardation were noted at 48 mg/kg/day which is approximately 23 times the MRHD of 20 mg on a mg/m2 basis. Slight maternal toxicity (clinical signs and decreased body weight gain and food consumption) was seen at this dose. Slightly increased offspring mortality was also seen at 24 mg/kg/day. The no-effect dose was 12 mg/kg/day which is approximately 6 times the MRHD of 20 mg on a mg/m2 basis.
In two rat embryo/fetal development studies, oral administration of racemic citalopram (32, 56, or 112 mg/kg/day) to pregnant animals during the period of organogenesis resulted in decreased embryo/fetal growth and survival and an increased incidence of fetal abnormalities (including cardiovascular and skeletal defects) at the high dose, which is approximately 18 times the MRHD of 60 mg/day on a mg/m2 basis. This dose was also associated with maternal toxicity (clinical signs, decreased body weight gain). The developmental no-effect dose was 56 mg/kg/day is approximately 9 times the MRHD on a mg/m2 basis. In a rabbit study, no adverse effects on embryo/fetal development were observed at doses of racemic citalopram of up to 16 mg/kg/day, or approximately 5 times the MRHD on a mg/m2 basis. Thus, developmental effects of racemic citalopram were observed at a maternally toxic dose in the rat and were not observed in the rabbit.
When female rats were treated with racemic citalopram (4.8, 12.8, or 32 mg/kg/day) from late gestation through weaning, increased offspring mortality during the first 4 days after birth and persistent offspring growth retardation were observed at the highest dose, which is approximately 5 times the MRHD of 60 mg on a mg/m2 basis. The no-effect dose was 12.8 mg/kg/day is approximately 2 times the MRHD on a mg/m2 basis. Similar effects on offspring mortality and growth were seen when dams were treated throughout gestation and early lactation at doses ≥ 24 mg/kg/day, approximately 4 times the MRHD on a mg/m2 basis. A no-effect dose was not determined in that study.8.2 Lactation
Risk Summary
Data from the published literature report the presence of escitalopram and desmethylescitalopram in human milk (see Data). There are reports of excessive sedation, restlessness, agitation, poor feeding and poor weight gain in infants exposed to escitalopram, through breast milk (see Clinical Considerations). There are no data on the effects of escitalopram or its metabolites on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for escitalopram oral solution and any potential adverse effects on the breastfed child from escitalopram oral solution or from the underlying maternal condition.
Clinical Considerations
Infants exposed to escitalopram oral solution should be monitored for excess sedation, restlessness, agitation, poor feeding and poor weight gain.
Data
A study of 8 nursing mothers on escitalopram with daily doses of 10 to 20 mg/day showed that exclusively breast-fed infants receive approximately 3.9% of the maternal weight-adjusted dose of escitalopram and 1.7% of the maternal weight-adjusted dose of desmethylcitalopram.
8.4 Pediatric Use
Major Depressive Disorder
The safety and effectiveness of escitalopram oral solution for the treatment of major depressive disorder have been established in pediatric patients 12 years of age and older. Use of escitalopram oral solution for this indication is supported by evidence from adequate and well-controlled studies in adults with additional evidence from an 8-week, flexible-dose, placebo-controlled study that compared escitalopram oral solution 10 mg to 20 mg once daily to placebo in pediatric patients 12 to 17 years of age with major depressive disorder [see Clinical Studies (14.1)]. The safety of escitalopram oral solution was similar to adult patients with MDD [see Adverse Reactions (6.1)].
The safety and effectiveness of escitalopram oral solution for the treatment of major depressive disorder have not been established in pediatric patients younger than 12 years of age. In a 24-week, open- label safety study in 118 pediatric patient (aged 7 to 11 years) who had major depressive disorder, the safety findings were consistent with the known safety and tolerability profile for escitalopram oral solution.
Generalized Anxiety Disorder
The safety and effectiveness of escitalopram oral solution for the treatment of generalized anxiety disorder have not been established in pediatric patients younger than 7 years of age.
Antidepressants increase the risk of suicidal thoughts and behaviors in pediatric patients [see Warnings and Precautions (5.1)]. Decreased appetite and weight loss have been observed in association with the use of SSRIs. Consequently, regular monitoring of weight and growth should be performed in children and adolescents treated with an SSRI such as escitalopram oral solution.
Juvenile Animal Toxicity Data
In a juvenile animal study, male and female rats were administered escitalopram at 5, 40, or 80 mg/kg/day by oral gavage from postnatal day (PND) 21 to PND 69. A delay in sexual maturation was observed in both males and females at ≥ 40 mg/kg/day with a No Observed Adverse Effect Level (NOAEL) of 5 mg/kg/day. This NOAEL was associated with plasma AUC levels less than those measured at the maximum recommended dose (MRHD) in pediatrics (20 mg). However, there was no effect on reproductive function. Increased motor activity (both ambulatory and fine movements) was observed in females prior to daily dosing at ≥ 40 mg/kg/day (3.5 times the MRHD based on AUC levels). A reversible disruption of learning and memory function was observed in males at 80 mg/kg/day with a NOAEL of 40 mg/kg/day, which was associated with an AUC level 3.5 times those measured at the MRHD in pediatrics. There was no effect on learning and memory function in treated female rats.
Additional pediatric use information is approved for AbbVie Inc.’s LEXAPRO (escitalopram) oral solution. However, due to AbbVie Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
8.5 Geriatric Use
Approximately 69 patients (6%) of the 1,144 patients receiving escitalopram in controlled trials of escitalopram oral solution in major depressive disorder and GAD were 60 years of age or older [see Clinical Studies (14.1, 14.2)]. The number of elderly patients in these trials was insufficient to adequately assess for possible differential efficacy and safety measures on the basis of age. Nevertheless, greater sensitivity of some elderly individuals to effects of escitalopram oral solution cannot be ruled out.
In two pharmacokinetic studies, escitalopram half-life was increased by approximately 50% in subjects 65 years and older as compared to young subjects and Cmax was unchanged [see Clinical Pharmacology (12.3)]. The recommended dosage of escitalopram oral solution for elderly patients is 10 mg daily [see Dosage and Administration (2.5)].
SSRIs, including escitalopram oral solution, have been associated with cases of clinically significant hyponatremia in elderly patients, who may be at greater risk for this adverse reaction [see Warnings and Precautions (5.6)].
Of 4,422 patients in clinical studies of racemic citalopram, 1,357 were 60 and over, 1,034 were 65 and over, and 457 were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the geriatric and younger patients, but again, greater sensitivity of some elderly individuals cannot be ruled out.
8.6 Hepatic Impairment
Increased citalopram exposure occurs in patients with hepatic impairment [see Clinical Pharmacology (12.3)]. The recommended dosage of escitalopram oral solution in patients with hepatic impairment is 10 mg daily [see Dosage and Administration (2.5)].
8.7 Renal Impairment
Pharmacokinetics of escitalopram oral solution in patients with a creatinine clearance less than 20 mL/minute has not been evaluated. No dosage adjustment is necessary for patients with mild or moderate renal impairment [see Dosage and Administration (2.5), Clinical Pharmacology (12.3)].
-
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse and Dependence
Physical and Psychological Dependence
Animal studies suggest that the abuse liability of racemic citalopram is low. Escitalopram oral solution has not been systematically studied in humans for its potential for abuse, tolerance, or physical dependence. The premarketing clinical experience with escitalopram oral solution did not reveal any drug-seeking behavior. However, these observations were not systematic and it is not possible to predict on the basis of this limited experience the extent to which a CNS-active drug will be misused, diverted, and/or abused once marketed. Consequently, physicians should carefully evaluate escitalopram oral solution patients for history of drug abuse and follow such patients closely, observing them for signs of misuse or abuse (e.g., development of tolerance, incrementations of dose, drug-seeking behavior). -
10 OVERDOSAGE
The following have been reported with escitalopram overdosage:
- Seizures, which may be delayed, and altered mental status including coma.
- Cardiovascular toxicity, which may be delayed, including QRS and QTc interval prolongation, wide complex tachyarrhythmias, and torsade de pointes. Hypertension most commonly seen, but rarely can see hypotension alone or with co-ingestants including alcohol.
- Serotonin syndrome (patients with a multiple drug overdosage with other proserotonergic drugs may have a higher risk).
Prolonged cardiac monitoring is recommended in escitalopram oral solution overdosage ingestions due to the arrhythmia risk.
Gastrointestinal decontamination with activated charcoal should be considered in patients who present early after a escitalopram oral solution overdose.
Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdose management recommendations. -
11 DESCRIPTION
Escitalopram oral solution, USP contains escitalopram a selective serotonin reuptake inhibitor (SSRI), present as escitalopram oxalate salt. Escitalopram is the pure S- enantiomer (single isomer) of the racemic bicyclic phthalane derivative citalopram. Escitalopram oxalate is designated S-(+)-1-[3(dimethyl-amino)propyl]-1-(p-fluorophenyl)-5-phthalancarbonitrile oxalate with the following structural formula:
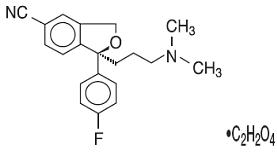
The molecular formula is C20H21FN2O C2H2O4 and the molecular weight is 414.40.
Escitalopram oxalate USP occurs as a fine, white to almost white, crystalline powder and is freely soluble in methanol and dimethyl sulfoxide (DMSO), soluble in isotonic saline solution, sparingly soluble in water and ethanol, slightly soluble in ethyl acetate, and insoluble in heptane.
Escitalopram oral solution USP contains 1.28 mg/mL escitalopram oxalate USP equivalent to 1 mg/mL escitalopram base. It also contains the following inactive ingredients: anhydrous citric acid, DL – malic acid, glycerin, methylparaben, natural peppermint flavor, noncrystalizing sorbitol solution, propylene glycol, propylparaben, purified water, and sodium citrate. -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of antidepressant action of escitalopram, the S-enantiomer of racemic citalopram, is presumed to be linked to potentiation of serotonergic activity in the central nervous system (CNS) resulting from its inhibition of CNS neuronal reuptake of serotonin (5-HT).
12.2 Pharmacodynamics
In vitro and in vivo studies in animals suggest that escitalopram is a highly selective serotonin reuptake inhibitor (SSRI) with minimal effects on norepinephrine and dopamine neuronal reuptake. Escitalopram is at least 100-fold more potent than the R-enantiomer with respect to inhibition of 5-HT reuptake and inhibition of 5-HT neuronal firing rate. Tolerance to a model of antidepressant effect in rats was not induced by long-term (up to 5 weeks) treatment with escitalopram. Escitalopram has no or very low affinity for serotonergic (5-HT1-7) or other receptors including alpha- and beta-adrenergic, dopamine (D1-5), histamine (H1-3), muscarinic (M1-5), and benzodiazepine receptors. Escitalopram also does not bind to, or has low affinity for, various ion channels including Na+, K+, Cl-, and Ca++ channels. Antagonism of muscarinic, histaminergic, and adrenergic receptors has been hypothesized to be associated with various anticholinergic, sedative, and cardiovascular side effects of other psychotropic drugs.
12.3 Pharmacokinetics
The single- and multiple-dose pharmacokinetics of escitalopram are linear and dose-proportional in a dose range of 10 to 30 mg/day.
With once-daily dosing, steady state plasma concentrations are achieved within approximately one week. At steady state, the extent of accumulation of escitalopram in plasma in young healthy subjects was 2.2 to 2.5 times the plasma concentrations observed after a single dose.
Absorption
The absolute bioavailability of citalopram is about 80% relative to an intravenous dose. The tablet and the oral solution dosage forms of escitalopram oxalate are bioequivalent.
Following a single oral dose (20 mg tablet or solution) of escitalopram, peak blood levels occur at about 5 hours. Absorption of escitalopram is not affected by food.
Distribution
The binding of escitalopram to human plasma proteins is approximately 56%. The volume of distribution of citalopram is about 12 L/kg. Data specific on escitalopram are unavailable.
Elimination
Biotransformation of escitalopram is mainly hepatic, with a mean terminal half-life of about 27 to 32 hours. The oral clearance of escitalopram is 600 mL/min, with approximately 7% of that due to renal clearance.
Metabolism
Escitalopram is metabolized to S-DCT and S-didemethylcitalopram (S-DDCT). In humans, unchanged escitalopram is the predominant compound in plasma. At steady state, the concentration of the escitalopram metabolite S-DCT in plasma is approximately one-third that of escitalopram. The level of S-DDCT was not detectable in most subjects. In vitro studies show that escitalopram is at least 7 and 27 times more potent than S-DCT and S-DDCT, respectively, in the inhibition of serotonin reuptake, suggesting that the metabolites of escitalopram do not contribute significantly to the antidepressant actions of escitalopram. S-DCT and S-DDCT also have no or very low affinity for serotonergic (5-HT1-7) or other receptors including alpha- and beta-adrenergic, dopamine (D1-5), histamine (H1-3), muscarinic (M1-5), and benzodiazepine receptors. S-DCT and S-DDCT also do not bind to various ion channels including Na+, K+, Cl-, and Ca++ channels. In vitro studies using human liver microsomes indicated that CYP3A4 and CYP2C19 are the primary isozymes involved in the N-demethylation of escitalopram.
Excretion
Following oral administrations of escitalopram, the fraction of drug recovered in the urine as escitalopram and S-demethylcitalopram (S-DCT) is about 8% and 10%, respectively.
Specific Populations
Pediatric Patients
Pediatric patients 12 to 17 years of age: In a single dose study of 10 mg escitalopram, AUC of escitalopram decreased by 19%, and Cmax increased by 26% in healthy pediatric subjects 12 to 17 years of age compared to adults. Following multiple dosing of 40 mg/day citalopram, escitalopram elimination half-life, steady-state Cmax and AUC were similar in pediatric patients 12 to 17 years of age with MDD compared to adults [see Use in Specific Populations (8.4)].
Geriatric Patients
Escitalopram pharmacokinetics in subjects ≥ 65 years of age were compared to adults in a single-dose and a multiple-dose study. Escitalopram AUC and half-life were increased by approximately 50% in elderly subjects, and Cmax was unchanged [see Dosage and Administration (2.5), Use in Specific Populations (8.5)].
Male and Female Patients
Based on data from single- and multiple-dose studies measuring escitalopram in elderly, young adults, and adolescents, no dosage adjustment on the basis of gender is needed.
Patients with Hepatic Impairment
Citalopram oral clearance was reduced by 37% and half-life was doubled in patients with reduced hepatic function compared to normal subjects [see Dosage and Administration (2.5), Use in Specific Populations (8.6)].
Patients with Renal Impairment
In patients with mild to moderate renal function impairment, oral clearance of citalopram was reduced by 17% compared to normal subjects. No information is available about the pharmacokinetics of escitalopram in patients with severely reduced renal function (creatinine clearance < 20 mL/min) [see Use in Specific Populations (8.7)].
Drug Interaction Studies
In vitro enzyme inhibition data did not reveal an inhibitory effect of escitalopram on CYP3A4, -1A2, -2C9, -2C19, and -2E1. Based on in vitro data, escitalopram would be expected to have little inhibitory effect on in vivo metabolism mediated by these cytochromes. While in vivo data to address this question are limited, results from drug interaction studies suggest that escitalopram, at a dose of 20 mg, has no 3A4 inhibitory effect and a modest 2D6 inhibitory effect [see Drug Interactions (7)].
CYP3A4 and CYP2C19 Inhibitors
In vitro studies indicated that CYP3A4 and -2C19 are the primary enzymes involved in the metabolism of escitalopram. However, coadministration of escitalopram (20 mg) and ritonavir (600 mg), a potent inhibitor of CYP3A4, did not significantly affect the pharmacokinetics of escitalopram. Because escitalopram is metabolized by multiple enzyme systems, inhibition of a single enzyme may not appreciably decrease escitalopram clearance.
Cimetidine
In subjects who had received 21 days of 40 mg/day racemic citalopram, combined administration of 400 mg twice a day cimetidine for 8 days resulted in an increase in citalopram AUC and Cmax of 43% and 39%, respectively. The clinical significance of these findings is unknown.
Digoxin
In subjects who had received 21 days of 40 mg/day racemic citalopram, combined administration of citalopram and digoxin (single dose of 1 mg) did not significantly affect the pharmacokinetics of either citalopram or digoxin.
Lithium
Coadministration of racemic citalopram (40 mg/day for 10 days) and lithium (30 mmol/day for 5 days) had no significant effect on the pharmacokinetics of citalopram or lithium. Plasma lithium levels should be monitored with appropriate adjustment to the lithium dose in accordance with standard clinical practice. Because lithium may enhance the serotonergic effects of escitalopram, caution should be exercised when escitalopram oral solution and lithium are coadministered.
Theophylline
Combined administration of racemic citalopram (40 mg/day for 21 days) and the CYP1A2 substrate theophylline (single dose of 300 mg) did not affect the pharmacokinetics of theophylline. The effect of theophylline on the pharmacokinetics of citalopram was not evaluated.
Ketoconazole
Combined administration of racemic citalopram (40 mg) and ketoconazole (200 mg), a potent CYP3A4 inhibitor, decreased the Cmax and AUC of ketoconazole by 21% and 10%, respectively, and did not significantly affect the pharmacokinetics of citalopram.
Ritonavir
Combined administration of a single dose of ritonavir (600 mg), both a CYP3A4 substrate and a potent inhibitor of CYP3A4, and escitalopram (20 mg) did not affect the pharmacokinetics of either ritonavir or escitalopram.
Triazolam
Combined administration of racemic citalopram (titrated to 40 mg/day for 28 days) and the CYP3A4 substrate triazolam (single dose of 0.25 mg) did not significantly affect the pharmacokinetics of either citalopram or triazolam.
Metoprolol
Administration of 20 mg/day escitalopram oral solution for 21 days in healthy volunteers resulted in a 50% increase in Cmax and 82% increase in AUC of the beta-adrenergic blocker metoprolol (given in a single dose of 100 mg). Increased metoprolol plasma levels have been associated with decreased cardioselectivity. Coadministration of escitalopram oral solution and metoprolol had no clinically significant effects on blood pressure or heart rate.
Alcohol
Escitalopram oral solution did not potentiate the cognitive and motor effects of alcohol in a clinical trial. As with other psychotropic medications, the use of alcohol by patients taking escitalopram oral solution is not recommended.
Warfarin
Administration of 40 mg/day racemic citalopram for 21 days did not affect the pharmacokinetics of warfarin, a CYP3A4 substrate. Prothrombin time was increased by 5%. The clinical significance of these findings is unknown.
Additional pediatric use information is approved for AbbVie Inc.’s LEXAPRO (escitalopram) oral solution. However, due to AbbVie Inc.’s marketing exclusivity rights, this drug product is not labeled with that information. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Racemic citalopram was administered in the diet to NMRI/BOM strain mice and COBS WI strain rats for 18 and 24 months, respectively. There was no evidence for carcinogenicity of racemic citalopram in mice receiving up to 240 mg/kg/day. There was an increased incidence of small intestine carcinoma in rats receiving 8 or 24 mg/kg/day racemic citalopram. A no-effect dose for this finding was not established. The relevance of these findings to humans is unknown.
Mutagenesis
Racemic citalopram was mutagenic in the in vitro bacterial reverse mutation assay (Ames test) in 2 of 5 bacterial strains (Salmonella TA98 and TA1537) in the absence of metabolic activation. It was clastogenic in the in vitro Chinese hamster lung cell assay for chromosomal aberrations in the presence and absence of metabolic activation. Racemic citalopram was not mutagenic in the in vitro mammalian forward gene mutation assay (HPRT) in mouse lymphoma cells or in a coupled in vitro/in vivo unscheduled DNA synthesis (UDS) assay in rat liver. It was not clastogenic in the in vitro chromosomal aberration assay in human lymphocytes or in two in vivo mouse micronucleus assays.
Impairment of Fertility
When racemic citalopram was administered orally to 16 male and 24 female rats prior to and throughout mating and gestation at doses of 32, 48, and 72 mg/kg/day, mating was decreased at all doses, and fertility was decreased at doses ≥ 32 mg/kg/day. Gestation duration was increased at 48 mg/kg/day.13.2 Animal Toxicology and/or Pharmacology
Retinal Changes in Rats
Pathologic changes (degeneration/atrophy) were observed in the retinas of albino rats in the 2-year carcinogenicity study with racemic citalopram. There was an increase in both incidence and severity of retinal pathology in both male and female rats receiving 80 mg/kg/day. Similar findings were not present in rats receiving 24 mg/kg/day of racemic citalopram for two years, in mice receiving up to 240 mg/kg/day of racemic citalopram for 18 months, or in dogs receiving up to 20 mg/kg/day of racemic citalopram for one year.
Additional studies to investigate the mechanism for this pathology have not been performed, and the potential significance of this effect in humans has not been established.
Cardiovascular Changes in Dogs
In a one-year toxicology study, 5 of 10 beagle dogs receiving oral racemic citalopram doses of 8 mg/kg/day died suddenly between weeks 17 and 31 following initiation of treatment. Sudden deaths were not observed in rats at doses of racemic citalopram up to 120 mg/kg/day, which produced plasma levels of citalopram and its metabolites demethylcitalopram and didemethylcitalopram (DDCT) similar to those observed in dogs at 8 mg/kg/day. A subsequent intravenous dosing study demonstrated that in beagle dogs, racemic DDCT caused QT prolongation, a known risk factor for the observed outcome in dogs. -
14 CLINICAL STUDIES
14.1 Major Depressive Disorder
Adults
The efficacy of escitalopram oral solution as a treatment for major depressive disorder was established in three, 8-week, placebo-controlled studies conducted in outpatients between 18 and 65 years of age who met DSM-IV criteria for major depressive disorder. The primary outcome in all three studies was change from baseline to endpoint in the Montgomery Asberg Depression Rating Scale (MADRS).
A fixed-dose study compared 10 mg daily escitalopram oral solution and 20 mg daily escitalopram oral solution to placebo and 40 mg daily citalopram. The 10 mg daily and 20 mg daily escitalopram oral solution treatment groups showed statistically significant greater mean improvement compared to placebo on the MADRS. The 10 mg and 20 mg escitalopram oral solution groups were similar on this outcome measure.
In a second fixed-dose study of 10 mg daily escitalopram oral solution and placebo, the 10 mg daily escitalopram oral solution treatment group showed statistically significant greater mean improvement compared to placebo on the MADRS.
In a flexible-dose study, comparing escitalopram oral solution, titrated between 10 mg and 20 mg daily, to placebo and citalopram, titrated between 20 mg and 40 mg daily, the escitalopram oral solution treatment group showed statistically significant greater mean improvement compared to placebo on the MADRS.
Analyses of the relationship between treatment outcome and age, gender, and race did not suggest any differential responsiveness on the basis of these patient characteristics.
In a longer-term trial, 274 patients meeting (DSM-IV) criteria for major depressive disorder, who had responded during an initial 8 week, open-label treatment phase with escitalopram oral solution 10 mg or 20 mg daily, were randomized to continuation of escitalopram oral solution at their same dose, or to placebo, for up to 36 weeks of observation for relapse. Response during the open-label phase was defined by having a decrease of the MADRS total score to ≤ 12. Relapse during the double-blind phase was defined as an increase of the MADRS total score to ≥ 22, or discontinuation due to insufficient clinical response. Patients receiving continued escitalopram oral solution experienced a statistically significant longer time to relapse compared to those receiving placebo.
Pediatric Patients 12 years of age and older
The efficacy of escitalopram oral solution as a treatment for major depressive disorder in pediatric patients 12 to 17 years was established in an 8-week, flexible-dose, placebo-controlled study that compared escitalopram oral solution (10 mg to 20 mg daily) to placebo in outpatients 12 to 17 years of age inclusive who met DSM-IV criteria for major depressive disorder (MDD). The primary outcome was change from baseline to endpoint in the Children’s Depression Rating Scale - Revised (CDRS-R). In this study, escitalopram oral solution showed statistically significant greater mean improvement compared to placebo on the CDRS-R.
The efficacy of escitalopram oral solution in the treatment of major depressive disorder in pediatric patients 12 to 17 years was established, in part, on the basis of extrapolation from the 8-week, flexible-dose, placebo-controlled study with racemic citalopram 20 mg to 40 mg daily. In this outpatient study in pediatric patients 7 to 17 years of age who met DSM-IV criteria for major depressive disorder, citalopram treatment showed statistically significant greater mean improvement from baseline, compared to placebo, on the CDRS-R; the positive results for this trial largely came from the 12 to 17 year subgroup.
Two additional flexible-dose, placebo-controlled MDD studies (one escitalopram oral solution study in patients ages 7 to 17 years and one citalopram study patients 13 to 18 years) did not demonstrate efficacy. The safety and effectiveness of escitalopram oral solution have not been established in pediatric patients less than 12 years of age with MDD.
14.2 Generalized Anxiety Disorder
Adults
The efficacy of escitalopram oral solution in the treatment of generalized anxiety disorder (GAD) in adults was demonstrated in three, 8-week, multicenter, flexible-dose, placebo-controlled studies that compared escitalopram oral solution (10 mg to 20 mg daily) to placebo in outpatients between 18 and 80 years of age who met DSM-IV criteria for GAD. In all three studies, escitalopram oral solution showed statistically significant greater mean improvement compared to placebo on the Hamilton Anxiety Scale (HAM-A).
There were too few patients in differing ethnic and age groups to adequately assess whether or not escitalopram oral solution has differential effects in these groups. There was no difference in response to escitalopram oral solution between men and women.
Additional pediatric use information is approved for AbbVie Inc.’s LEXAPRO (escitalopram) oral solution. However, due to AbbVie Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Oral Solution
Escitalopram Oral Solution USP 5 mg/5 mL is a clear, colorless peppermint flavored liquid.
240 mL Bottle NDC: 16571-769-24
Storage and Handling
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Suicidal Thoughts and Behaviors
Advise patients, their families and caregivers to look for the emergence of suicidal ideation and behavior, especially during treatment and when the dose is adjusted up or down, and instruct them to report such symptoms to their healthcare provider [see Boxed Warning and Warnings and Precautions (5.1)].
Serotonin Syndrome
Caution patients about the risk of serotonin syndrome, particularly with the with the concomitant use of escitalopram oral solution with other serotonergic drugs including triptans, tricyclic antidepressants, opioids, lithium, tryptophan, buspirone, amphetamines, and St. John’s Wort, and with drugs that impair metabolism of serotonin (in particular, MAOIs, both those intended to treat psychiatric disorders and also others, such as linezolid). Instruct patients to contact their health care provider or report to the emergency room if they experience signs or symptoms of serotonin syndrome [see Warnings and Precautions (5.2), Drug Interactions (7)].
Discontinuation Syndrome
Advise patients not to abruptly discontinue escitalopram oral solution and to discuss any tapering regimen with their healthcare provider. Inform patients that adverse reactions can occur when escitalopram oral solution is discontinued [see Warnings and Precautions (5.3)].
Activation of Mania or Hypomania
Advise patients and their caregivers to observe for signs of activation of mania/hypomania and instruct them to report such symptoms to the healthcare provider [see Warnings and Precautions (5.5)].
Increased Risk of Bleeding
Inform patients about the concomitant use of escitalopram oral solution with NSAIDs, aspirin, warfarin, other antiplatelet drugs, or other anticoagulants because the combined use has been associated with an increased risk of bleeding. Advise patients to inform their healthcare providers if they are taking or planning to take any prescription or over-the-counter medications that increase the risk of bleeding [see Warnings and Precautions (5.7)].
Angle Closure Glaucoma
Advise patients that taking escitalopram oral solution can cause mild pupillary dilation, which in susceptible individuals, can lead to an episode of angle closure glaucoma. Pre-existing glaucoma is almost always open-angle glaucoma because angle closure glaucoma, when diagnosed, can be treated definitively with iridectomy. Open-angle glaucoma is not a risk factor for angle closure glaucoma.
Patients may wish to be examined to determine whether they are susceptible to angle closure, and have a prophylactic procedure (e.g., iridectomy), if they are susceptible [see Warnings and Precautions (5.9)].
Sexual Dysfunction
Advise patients that use of escitalopram oral solution may cause symptoms of sexual dysfunction in both male and female patients. Inform patients that they should discuss any changes in sexual function and potential management strategies with their healthcare provider [see Warnings and Precautions (5.11)].
Concomitant Medications
Since escitalopram is the active isomer of racemic citalopram (Celexa), the two agents should not be coadministered. Patients should be advised to inform their physician if they are taking, or plan to take, any prescription or over-the-counter drugs, as there is a potential for interactions.
Interference with Psychomotor Performance
Because psychoactive drugs may impair judgment, thinking, or motor skills, patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that escitalopram oral solution therapy does not affect their ability to engage in such activities.
Alcohol
Patients should be told that, although escitalopram oral solution has not been shown in experiments with normal subjects to increase the mental and motor skill impairments caused by alcohol, the concomitant use of escitalopram oral solution and alcohol in depressed patients is not advised.
Pregnancy
Advise pregnant women to notify their healthcare providers if they become pregnant or intend to become pregnant during treatment with escitalopram oral solution.
Advise patients that escitalopram oral solution use later in pregnancy may lead to increased risk for neonatal complications requiring prolonged hospitalization, respiratory support, tube feeding, and/or persistent pulmonary hypertension (PPHN) of the newborn [see Use in Specific Populations (8.1)].
Advise women that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to escitalopram oral solution during pregnancy [see Use in Specific Populations (8.1)].
Lactation
Advise breastfeeding women using escitalopram oral solution to monitor infants for excess sedation, restlessness, agitation, poor feeding and poor weight gain and to seek medical care if they notice these signs [see Use in Specific Populations (8.2)].
The brands listed are trademarks of their respective owners and are not trademarks of Aurobindo Pharma Limited.Dispense with Medication Guide available at: http://www.risingpharma.com/med-guides.html
Distributed by:
Rising Pharma Holdings, Inc.
East Brunswick, NJ 08816
Made in India
Code: TS/DRUGS/19/1993
Revised: 05/2024 -
MEDICATION GUIDE
Dispense with Medication Guide available at: http://www.risingpharma.com/med-guides.html
MEDICATION GUIDE
Escitalopram (es'' sye tal' oh pram)
Oral Solution USP
What is the most important information I should know about escitalopram oral solution?
Escitalopram oral solution may cause serious side effects, including:
- Increased risk of suicidal thoughts or actions. Escitalopram oral solution and other antidepressant medicines increase the risk of suicidal thoughts and actions in people 24 years of age and younger, especially within the first few months of treatment or when the dose is changed.
- Depression or other mental illnesses are the most important causes of suicidal thoughts or actions.
- Pay close attention to any changes, especially sudden changes in mood, behavior, thoughts, or feelings, or if you or your child develop suicidal thoughts or actions. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call your healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings or if you or your child develop suicidal thoughts or actions.
- Keep all follow-up visits with your healthcare provider as scheduled and call your healthcare provider between visits if you are worried about symptoms.
- attempts to commit suicide
- acting on dangerous impulses
- acting aggressive, being angry or violent
- thoughts about suicide or dying
- new or worse depression
- new or worsening anxiety
- panic attacks
- feeling very agitated or restless
- new or worse irritability
- trouble sleeping
- an extreme increase in activity or talking (mania)
- other unusual changes in behavior or mood
What is escitalopram oral solution?
Escitalopram oral solution is a prescription medicine used to treat:
- a certain type of depression called Major Depressive Disorder (MDD) in adults and children 12 years of age and older
- Generalized Anxiety Disorder (GAD) in adults
It is not known if escitalopram oral solution is safe and effective for use in children under 12 years of age with MDD or children under 7 years of age with GAD.
Do not take escitalopram oral solution if you or your child:
- are taking, or have stopped taking within the last 14 days, a medicine called a monoamine oxidase inhibitor (MAOI), including the antibiotic linezolid or intravenous methylene blue
- are taking the antipsychotic medicine pimozide
- are allergic to escitalopram or citalopram or any of the ingredients in escitalopram oral solution. See the end of this Medication Guide for a complete list of ingredients in escitalopram oral solution.
Ask your healthcare provider or pharmacist if you are not sure if you or your child take an MAOI, including the antibiotic linezolid or intravenous methylene blue.
Do not start taking an MAOI for at least 14 days after you or your child have stopped treatment with escitalopram oral solution.
Before taking escitalopram oral solution, tell your healthcare provider about all your medical conditions, including if you or your child:
- have or had seizures or convulsions
- have, or have a family history of bipolar disorder, mania, or hypomania
- have low blood sodium levels
- have or had bleeding problems
- have high pressure in the eye (glaucoma)
- have heart, liver, or kidney problems
- are pregnant or plan to become pregnant. Escitalopram oral solution may harm the unborn baby. Taking escitalopram oral solution during the third trimester of pregnancy may cause the baby to have withdrawal symptoms, or breathing, temperature control, feeding, or other problems after birth. Talk to your healthcare provider about the risks to the baby if you or your child take escitalopram oral solution during pregnancy.
- Tell your healthcare provider right away if you or your child become pregnant or think you may be pregnant during treatment with escitalopram oral solution.
- There is a pregnancy registry for females who are exposed to escitalopram oral solution during pregnancy. The purpose of the registry is to collect information about the health of females exposed to escitalopram oral solution and their baby. If you or your child become pregnant during treatment with escitalopram oral solution, talk to your healthcare provider about registering with the National Pregnancy Registry for Antidepressants at 1-844-405-6185 or visit online at https://womensmentalhealth.org/research/pregnancyregistry/antidepressants.
- are breastfeeding or plan to breastfeed. Escitalopram passes into breast milk and may harm the baby. Talk to your healthcare provider about the best way to feed the baby during treatment with escitalopram oral solution.
- If you or your child breastfeed during treatment with escitalopram oral solution, call your healthcare provider if the baby develops sleepiness or fussiness, or is not feeding or gaining weight well.
Escitalopram oral solution and some medicines may affect each other and may cause serious side effects.
Escitalopram oral solution may affect the way other medicines work and other medicines may affect the way escitalopram oral solution works.
Especially tell your healthcare provider if you take:
- medicines used to treat migraine headache known as triptans
- tricyclic antidepressants
- lithium
- tramadol, fentanyl, meperidine, methadone, or other opioids
- tryptophan
- buspirone
- amphetamines
- St. John’s Wort
- medicines used to treat mood, anxiety, psychotic or thought disorders, including selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs)
- diuretics
- medicines that can affect blood clotting such as aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs) and warfarin
Ask your healthcare provider if you are not sure if you or your child are taking any of these medicines. Your healthcare provider can tell you if it is safe to take escitalopram oral solution with your other medicines.
Do not start or stop any other medicines during treatment with escitalopram oral solution without talking to your healthcare provider first. Stopping escitalopram oral solution suddenly may cause you or your child to have serious side effects. See, “What are the possible side effects of escitalopram oral solution?”
Know the medicines you or your child take. Keep a list of them to show your healthcare provider and pharmacist when you get new medicine.
How should I take escitalopram oral solution?
- Take escitalopram oral solution exactly as prescribed. Your healthcare provider may need to change the dose of escitalopram oral solution until it is the right dose for you or your child.
- Take escitalopram oral solution 1 time each day, in the morning or the evening.
- Take escitalopram oral solution with or without food.
- If you or your child take too much escitalopram oral solution, call your healthcare provider or Poison Help Line at 1-800-222-1222, or go to the nearest hospital emergency room right away.
What should I avoid while taking escitalopram oral solution?
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how escitalopram oral solution affects you. Escitalopram oral solution can cause sleepiness or may affect your ability to make decisions, think clearly, or react quickly.
- Do not drink alcohol during treatment with escitalopram oral solution.
What are the possible side effects of escitalopram oral solution?
Escitalopram oral solution may cause serious side effects, including:
- See “What is the most important information I should know about escitalopram oral solution?”
-
Serotonin syndrome. A potentially life-threatening problem called serotonin syndrome can happen when escitalopram oral solution is taken with certain other medicines. See “Do not take escitalopram oral solution if you?” Call your healthcare provider or go to the nearest hospital emergency room right away if you or your child have any of the following signs and symptoms of serotonin syndrome:
- agitation
- seeing or hearing things that are not real (hallucinations)
- confusion
- coma
- fast heartbeat
- blood pressure changes
- sweating
- shaking (tremors), stiff muscles, or muscle twitching
- flushing
- dizziness
- seizures
- high body temperature (hyperthermia)
- nausea, vomiting, diarrhea
- loss of coordination
-
Discontinuation syndrome. Suddenly stopping escitalopram oral solution may cause you or your child to have serious side effects. Your healthcare provider may want to decrease the dose slowly. Symptoms may include:
- changes in mood
- headache
- irritability and agitation
- tiredness
- dizziness
- problems sleeping
- electric shock sensation (paresthesia)
- hypomania
- anxiety
- ringing in your ears (tinnitus)
- confusion
- seizures
- Seizures (convulsions).
-
Manic episodes. Manic episodes may happen in people with bipolar disorder who take escitalopram oral solution. Symptoms may include:
- greatly increased energy
- severe trouble sleeping
- racing thoughts
- reckless behavior
- unusually grand ideas
- excessive happiness or irritability
- talking more or faster than usual
-
Low sodium levels in the blood (hyponatremia). Low sodium levels in the blood that may be serious and may cause death can happen during treatment with escitalopram oral solution. Elderly people and people who take certain medicines may be at greater risk for developing low sodium levels in the blood. Signs and symptoms may include:
- headache
- problems concentrating or thinking
- weakness or feeling unsteady which can lead to falls
- confusion
- memory problems
In more severe or more sudden cases, signs and symptoms include:
- seeing or hearing things that are not real (hallucinations)
- fainting
- seizures
- coma
- stopping breathing (respiratory arrest)
- Increased risk of bleeding: Taking escitalopram oral solution with aspirin, NSAIDS, warfarin, or other blood thinners may add to this risk. Tell your healthcare provider if you have any unusual bleeding or bruising.
-
Visual problems (angle-closure glaucoma). Escitalopram oral solution may cause a type of eye problem called angle-closure glaucoma in people with certain eye problems. You or your child may want to undergo an eye examination to see if you are at risk and receive preventative treatment if you are. Call your healthcare provider if you or your child have:
- eye pain
- changes in vision
- swelling or redness in or around the eye
- Sexual problems (dysfunction). Taking escitalopram oral solution may cause sexual problems.
- delayed ejaculation or inability to have an ejaculation
- decreased sex drive
- problems getting or keeping an erection
- decreased sex drive
- delayed orgasm or inability to have an orgasm
The most common side effects of escitalopram oral solution include:
- trouble sleeping
- sweating
- decreased sex drive
- delayed ejaculation
- tiredness
- delayed orgasm or inability to have an orgasm
- nausea
- sleepiness
These are not all the possible side effects of escitalopram oral solution.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store escitalopram oral solution?
- Store escitalopram oral solution at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep escitalopram oral solution and all medicines out of the reach of children.
General information about the safe and effective use of escitalopram oral solution.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use escitalopram oral solution for a condition for which it was not prescribed. Do not give escitalopram oral solution to other people, even if they have the same symptoms that you have. It may harm them. You may ask your pharmacist or healthcare provider for information about escitalopram oral solution that is written for health professionals.
What are the ingredients in escitalopram oral solution?
Active ingredient: escitalopram oxalate
Inactive ingredients: anhydrous citric acid, DL – malic acid, glycerin, methylparaben, natural peppermint flavor, noncrystalizing sorbitol solution, propylene glycol, propylparaben, purified water, and sodium citrate.
Distributed by:
Rising Pharma Holdings, Inc.
East Brunswick, NJ 08816
Made in India
Code: TS/DRUGS/19/1993
For more information, call Rising Pharma Holdings, Inc. at 1(844) 874-7464.
Additional pediatric use information is approved for AbbVie Inc.’s LEXAPRO (escitalopram) oral solution. However, due to AbbVie Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
The brands listed are trademarks of their respective owners and are not trademarks of Aurobindo Pharma Limited.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: 05/2024
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 5 mg/5 mL (240 mL Bottle)
Rising Pharma Holdings, Inc.
NDC: 16571-769-24
Escitalopram Oral
Solution USP
5 mg/5 mL
Pharmacist: Dispense with
Medication Guide.
240 mL Rx only
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 5 mg/5 mL (240 mL Carton Label)
Rising Pharma Holdings, Inc.
NDC: 16571-769-24
Escitalopram Oral
Solution USP
5 mg/5 mL
Pharmacist: Dispense with
Medication Guide.
240 mL Rx only
-
INGREDIENTS AND APPEARANCE
ESCITALOPRAM OXALATE
escitalopram oxalate solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 16571-769 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ESCITALOPRAM OXALATE (UNII: 5U85DBW7LO) (ESCITALOPRAM - UNII:4O4S742ANY) ESCITALOPRAM 5 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) MALIC ACID (UNII: 817L1N4CKP) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) PEPPERMINT (UNII: V95R5KMY2B) SORBITOL (UNII: 506T60A25R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) Product Characteristics Color Score Shape Size Flavor PEPPERMINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 16571-769-24 240 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/03/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA079062 04/03/2012 Labeler - Rising Pharma Holdings, Inc. (116880195) Registrant - Aurobindo Pharma Limited (650082092) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 918917642 ANALYSIS(16571-769) , MANUFACTURE(16571-769)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.