REVIVE SCIENCE Eczema Cream
REVIVE SCIENCE Eczema by
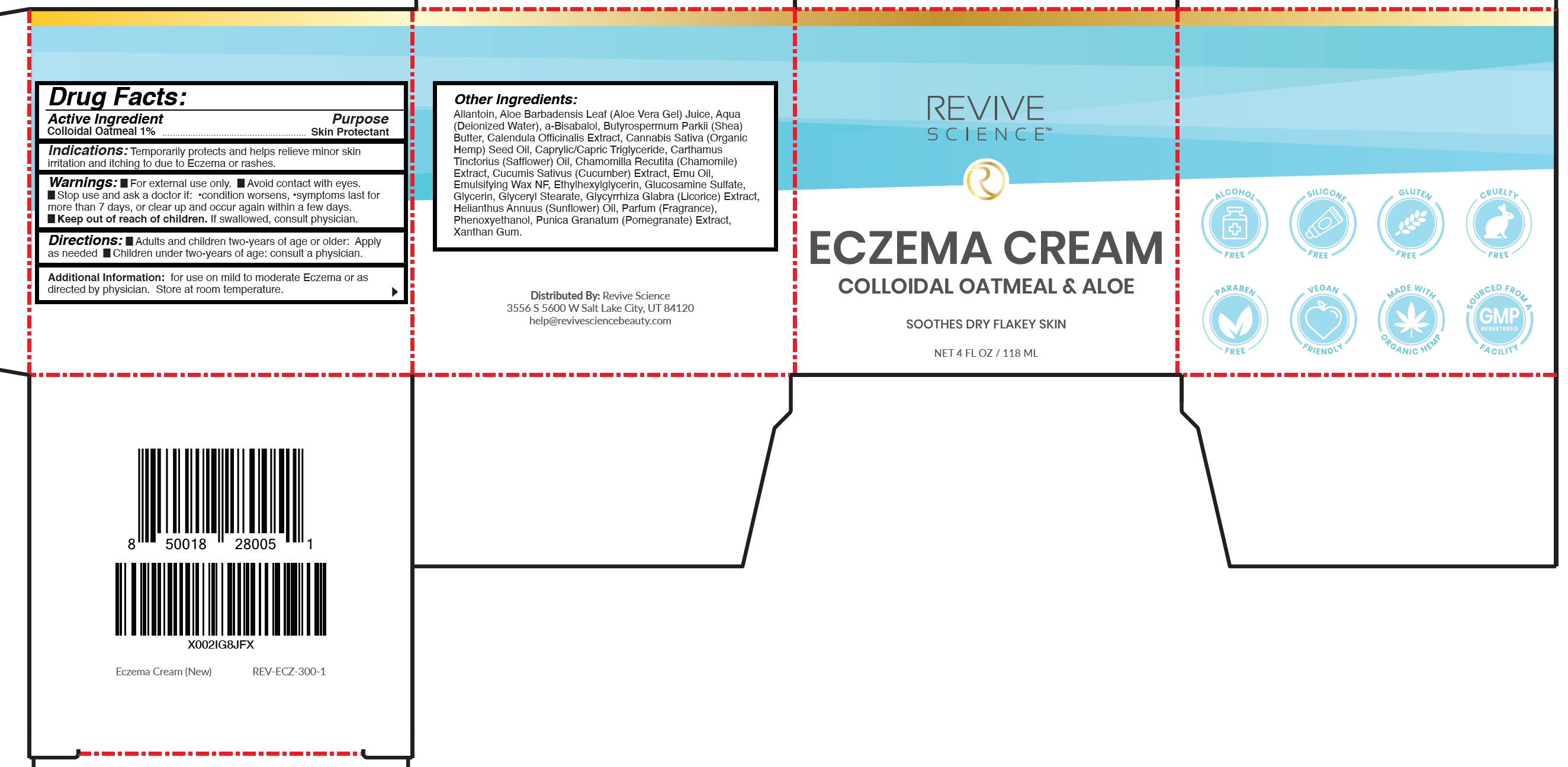
Drug Labeling and Warnings
REVIVE SCIENCE Eczema by is a Otc medication manufactured, distributed, or labeled by Revive Science, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
REVIVE SCIENCE ECZEMA- oatmeal cream
Revive Science, LLC
----------
REVIVE SCIENCE Eczema Cream
Indications:
Temporarily protects and helps relieve minor skin irritation and itching to due to Eczema or rashes.
Warnings:
- For external use only.
- Avoid contact with eyes.
Directions:
- Adults and children two-years of age or older: Apply as needed
- Children under two-years of age: consult a physician.
Additional Information:
for use on mild to moderate Eczema or as directed by physician. Store at room temperature.
Other Ingredients:
Allantoin, Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Aqua (Deionized Water), a-Bisabalol, Butyrospermum Parkii (Shea) Butter, Calendula Officinalis Extract, Cannabis Sativa (Organic Hemp) Seed Oil, Caprylic/Capric Triglyceride, Carthamus Tinctorius (Safflower) Oil, Chamomilla Recutita (Chamomile) Extract, Cucumis Sativus (Cucumber) Extract, Emu Oil, Emulsifying Wax NF, Ethylhexylglycerin, Glucosamine Sulfate, Glycerin, Glyceryl Stearate, Glycyrrhiza Glabra (Licorice) Extract, Helianthus Annuus (Sunflower) Oil, Parfum (Fragrance), Phenoxyethanol, Punica Granatum (Pomegranate) Extract, Xanthan Gum.
| REVIVE SCIENCE ECZEMA
oatmeal cream |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Revive Science, LLC (117480030) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.