PEG-3350 AND ELECTROLYTES- polyethylene glycol 3350, sodium sulfate anhydrous, sodium bicarbonate, sodium chloride, potassium chloride powder, for solution
PEG-3350 and Electrolytes by
Drug Labeling and Warnings
PEG-3350 and Electrolytes by is a Prescription medication manufactured, distributed, or labeled by A-S Medication Solutions. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PEG-3350 and Electrolytes for Oral Solution safely and effectively. See full prescribing information for PEG-3350 and Electrolytes for Oral Solution.
Polyethylene glycol 3350 and electrolytes for oral solution
Initial U.S. Approval: 1984RECENT MAJOR CHANGES
Warnings and Precautions, Aspiration: ( 5.7) 05/2021 INDICATIONS AND USAGE
PEG-3350 and Electrolytes for Oral Solution is a combination of PEG 3350, an osmotic laxative, and electrolytes indicated for cleansing of the colon in preparation for colonoscopy and barium enema X-ray examination in adults ( 1)
DOSAGE AND ADMINISTRATION
Preparation and Administration ( 2.1):
- Correct fluid and electrolyte abnormalities before treatment with PEG-3350 and Electrolytes for
Oral Solution. - Reconstitute PEG-3350 and Electrolytes for Oral Solution with water prior to ingestion.
- Do not take oral medications within 1 hour before the start or during administration of PEG-3350 and Electrolytes for Oral Solution. ( 2.1)
- Do not take other laxatives while taking PEG-3350 and Electrolytes for Oral Solution.
- Consume only clear liquids; avoid red and purple liquids.
- Consume water or other clear liquids up until 2 hours before the time of the colonoscopy.
- Do not consume solid food within 2 hours before starting PEG-3350 and Electrolytes for Oral Solution.
Adult Dosing Regimen ( 2.2):
- On day prior to colonoscopy, instruct patients to consume a light breakfast at least 2 hours before starting PEG-3350 and Electrolytes for Oral Solution.
- Begin the recommended dosage regimen for PEG-3350 and Electrolytes for Oral Solution early in the evening on the day before colonoscopy.
- Drink reconstituted solution at a rate of 8 ounces every 10 minutes, until 4 liters are consumed, or rectal effluent is clear.
- For complete information on dosing, preparation and administration, see the full prescribing information. ( 2.1, 2.2)
DOSAGE FORMS AND STRENGTHS
For Oral Solution: 236 g polyethylene glycol 3350, 22.74 g sodium sulfate (anhydrous), 6.74 g sodium bicarbonate, 5.86 g sodium chloride, 2.97 g potassium chloride and 3 g flavoring ingredients per 4 liters. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Risk of fluid and electrolyte abnormalities: Encourage adequate hydration, assess concurrent medications, and consider laboratory assessments prior to and after use. ( 5.1, 5.2, 7.1)
- Cardiac arrhythmias: Consider pre-dose and post-colonoscopy ECGs in patients at increased risk of serious cardiac arrhythmias. ( 5.2)
- Seizures: Use caution in patients with a history of seizures and patients at increased risk of seizure, including medications that lower the seizure threshold. ( 5.3, 7.1)
- Patients with renal impairment or taking concomitant medications that affect renal function: Use caution, ensure adequate hydration and consider testing. ( 5.4, 7.1, 8.6)
- Mucosal ulcerations: Consider potential for mucosal ulcerations when interpreting colonoscopy findings in patients with known or suspected inflammatory bowel disease. ( 5.5, 7.3)
- Patients at risk for aspiration: Observe during administration. ( 5.7)
- Hypersensitivity reactions including anaphylaxis: Inform patients to seek immediate medical care if symptoms occur. ( 5.8)
ADVERSE REACTIONS
DRUG INTERACTIONS
Some drugs increase risks due to fluid and electrolyte changes ( 7.1)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 6/2023
- Correct fluid and electrolyte abnormalities before treatment with PEG-3350 and Electrolytes for
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Preparation and Administration Instructions
2.2 Dosage Regimen
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Fluid and Serum Chemistry Abnormalities
5.2 Cardiac Arrhythmias
5.3 Seizures
5.4 Renal Impairment
5.5 Colonic Mucosal Ulcerations and Ischemic Colitis
5.6 Use in Patients with Significant Gastrointestinal Disease
5.7 Aspiration
5.8 Hypersensitivity Reactions
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Drugs that May Increase Risks Due to Fluid and Electrolyte Abnormalities
7.2 Potential for Reduced Drug Absorption
7.3 Stimulant Laxatives
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Preparation and Administration Instructions
- Correct fluid and electrolyte abnormalities before treatment with PEG-3350 and Electrolytes for Oral Solution [see Warnings and Precautions (5.1)].
- Reconstitute PEG-3350 and Electrolytes for Oral Solution with water prior to ingestion, do not take undissolved PEG-3350 and Electrolytes for Oral Solution [see Dosage and Administration (2.2)].
- Do not reconstitute with other liquids and/or add starch-based thickeners to the mixing container [see Warnings and Precautions (5.7)].
- Do not take oral medications within 1 hour before the start of or during administration of PEG-3350 and Electrolytes for Oral Solution [see Drug Interactions (7.2)].
- Do not take other laxatives while taking PEG-3350 and Electrolytes for Oral Solution [see Drug Interactions (7.3)].
- Consume only clear liquids, avoid red and purple liquids.
- Patients may consume water or other clear liquids during the bowel preparation and after completion of the bowel preparation up until 2 hours before the time of the colonoscopy.
- The solution is more palatable if chilled prior to administration.
- Do not consume solid food within 2 hours before starting PEG-3350 and Electrolytes for Oral Solution. For the best results, do not consume solid food for 3 to 4 hours before drinking the solution.
- If severe bloating, distention or abdominal pain occurs, slow or temporarily discontinue PEG-3350 and Electrolytes for Oral Solution until the symptoms abate.
2.2 Dosage Regimen
Instruct adult patients that on the day before the colonoscopy procedure, they may consume a light breakfast at least 2 hours before starting PEG-3350 and Electrolytes for Oral Solution. Begin the recommended dosage regiment for PEG-3350 and Electrolytes for Oral Solution early in the evening on the day before colonoscopy.
Instruct patients to take PEG-3350 and Electrolytes for Oral Solution in conjunction with clear liquids as follows:
4 Liter Jug
- Fill the supplied container containing the PEG-3350 and Electrolytes for Oral Solution powder with lukewarm drinking water to the 4-liter fill line
Do not add any other ingredients, flavors, etc. - After capping the container, shake vigorously several times to ensure that the ingredients are dissolved.
- Drink at a rate of 8 ounces every 10 minutes until the entire contents are consumed or the rectal effluent is clear. A loose watery bowel movement should result in approximately one hour.
- After reconstitution, keep solution refrigerated 2° to 8°C (36° to 46°F). Do not freeze. Use within 48 hours, discard unused portion.
Administration via a Nasogastric Tube
For patients with a nasogastric tube, administer the reconstituted PEG-3350 and Electrolytes for Oral Solution at a rate of 20 to 30 mL per minute (1.2 to 1.8 liters per hour).
-
3 DOSAGE FORMS AND STRENGTHS
For Oral Solution: 236 g polyethylene glycol 3350, 22.74 g sodium sulfate (anhydrous), 6.74 g sodium bicarbonate, 5.86 g sodium chloride and 2.97 g potassium chloride as a white powder. When reconstituted with water to a volume of 4 liters, the solution contains 59 g/L PEG-3350, 5.69 g/L sodium sulfate, 1.69 g/L sodium bicarbonate, 1.47 g/L sodium chloride and 0.743 g/L potassium chloride.
-
4 CONTRAINDICATIONS
PEG-3350 and Electrolytes for Oral Solution is contraindicated in the following conditions:
- Gastrointestinal (GI) obstruction [see Warnings and Precautions ( 5.6)]
- Bowel perforation [see Warnings and Precautions ( 5.6)]
- Toxic colitis or toxic megacolon
- Gastric retention
- Ileus
- Hypersensitivity to any component of PEG-3350 and Electrolytes for Oral Solution [see Warnings and Precautions ( 5.8)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Fluid and Serum Chemistry Abnormalities
Advise patients to hydrate adequately before, during, and after the use of PEG-3350 and Electrolytes for Oral Solution. Use caution in patients with congestive heart failure when replacing fluids. If a patient develops significant vomiting or signs of dehydration including signs of orthostatic hypotension after taking PEG-3350 and Electrolytes for Oral Solution, consider performing post-colonoscopy lab tests (electrolytes, creatinine, and BUN) and treat accordingly. Fluid and electrolyte disturbances can lead to serious adverse events including cardiac arrhythmias, seizures and renal impairment. Correct fluid and electrolyte abnormalities before treatment with PEG-3350 and Electrolytes for Oral Solution.
In addition, use caution when prescribing PEG-3350 and Electrolytes for Oral Solution for patients who have conditions, or who are using medications, that increase the risk for fluid and electrolyte disturbances or may increase the risk of adverse events of seizure, arrhythmias, and renal impairment [see Drug Interactions ( 7.1)] .
5.2 Cardiac Arrhythmias
There have been rare reports of serious arrhythmias associated with the use of ionic osmotic laxative products for bowel preparation. Use caution when prescribing PEG-3350 and Electrolytes for Oral Solution for patients at increased risk of arrhythmias (e.g., patients with a history of prolonged QT, uncontrolled arrhythmias, recent myocardial infarction, unstable angina, congestive heart failure, or cardiomyopathy). Consider pre-dose and post-colonoscopy ECGs in patients at increased risk of serious cardiac arrhythmias.
5.3 Seizures
There have been reports of generalized tonic-clonic seizures and/or loss of consciousness associated with use of bowel preparation products in patients with no prior history of seizures. The seizure cases were associated with electrolyte abnormalities (e.g., hyponatremia, hypokalemia, hypocalcemia, and hypomagnesemia) and low serum osmolality. The neurologic abnormalities resolved with correction of fluid and electrolyte abnormalities.
Use caution when prescribing PEG-3350 and Electrolytes for Oral Solution for patients with a history of seizures and in patients at increased risk of seizure, such as patients taking medications that lower the seizure threshold (e.g., tricyclic antidepressants), patients withdrawing from alcohol or benzodiazepines, or patients with known or suspected hyponatremia [see Drug Interactions ( 7.1)] .
5.4 Renal Impairment
Use caution when prescribing PEG-3350 and Electrolytes for Oral Solution for patients with impaired renal function or patients taking concomitant medications that may affect renal function (such as diuretics, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or non-steroidal anti-inflammatory drugs) [see Drug Interactions ( 7.1)] . Advise these patients of the importance of adequate hydration and consider performing baseline and post-colonoscopy laboratory tests (electrolytes, creatinine, and BUN) in these patients [see Use in Specific Populations ( 8.6)] .
5.5 Colonic Mucosal Ulcerations and Ischemic Colitis
Administration of osmotic laxative products may produce colonic mucosal aphthous ulcerations, and there have been reports of more serious cases of ischemic colitis requiring hospitalization. Concurrent use of stimulant laxatives and PEG-3350 and Electrolytes for Oral Solution may increase this risk [see Drug Interactions ( 7.3)] . Consider the potential for mucosal ulcerations resulting from the bowel preparation when interpreting colonoscopy findings in patients with known or suspect inflammatory bowel disease (IBD).
5.6 Use in Patients with Significant Gastrointestinal Disease
If gastrointestinal obstruction or perforation is suspected, perform appropriate diagnostic studies to rule out these conditions before administering PEG-3350 and Electrolytes for Oral Solution [see Contraindications ( 4)] . Use with caution in patients with severe active ulcerative colitis.
5.7 Aspiration
Use with caution in patients with impaired gag reflex, unconscious, or semiconscious patients, and patients prone to regurgitation or aspiration. Observe these patients during administration of PEG-3350 and Electrolytes for Oral Solution, especially if it is administered via nasogastric tube.
Do not combine PEG-3350 and Electrolytes for Oral Solution with starch-based thickeners [see Dosage and Administration ( 2.1)] . Polyethylene glycol (PEG), a component of PEG-3350 and Electrolytes for Oral Solution, when mixed with starch-thickened liquids reduces the viscosity of the starch-thickened liquid. When a PEG-based product used for another indication was mixed in starch-based pre-thickened liquids used in patients with dysphagia, thinning of the liquid occurred and cases of choking and potential aspiration were reported.
5.8 Hypersensitivity Reactions
PEG-3350 and Electrolytes for Oral Solution contains PEG and may cause serious hypersensitivity reactions including anaphylaxis, angioedema, rash, urticaria, and pruritus [see Adverse Reactions ( 6)] . Inform patients of the signs and symptoms of anaphylaxis and instruct them to seek immediate medical care should signs and symptoms occur.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Renal impairment [see Warnings and Precautions ( 5.4)]
- Colonic mucosal ulcerations and ischemic colitis [see Warnings and Precautions ( 5.5)]
- Patients with significant gastrointestinal disease [see Warnings and Precautions ( 5.6)]
- Aspiration [see Warnings and Precautions ( 5.7)]
The following adverse reactions associated with the use of PEG-3350 and Electrolytes for Oral Solution were identified in clinical trials or postmarketing reports. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency, reliably, or establish a causal relationship to drug exposure.
- Cardiovascular: arrhythmia, atrial fibrillation, peripheral edema, asystole, and acute pulmonary edema after aspiration [see Warnings and Precautions ( 5.2)] .
- Nervous system: tremor, seizure [see Warnings and Precautions ( 5.3)]
- Hypersensitivity: Urticaria/rash, pruritus, dermatitis, rhinorrhea, dyspnea, chest and throat tightness, fever, angioedema, anaphylaxis and anaphylactic shock [see Contraindications ( 4), Warnings and Precautions ( 5.8)]
- Gastrointestinal: Nausea, abdominal fullness and bloating are the most common adverse reactions (occurred in up to 50% of patients). Other less common adverse reactions include: abdominal cramps, vomiting, “butterfly-like” infiltrates on chest X-ray after vomiting and aspirating PEG, anal irritation, and upper GI bleeding from Mallory-Weiss Tear, esophageal perforation [usually with gastroesophageal reflux disease (GERD)].
-
7 DRUG INTERACTIONS
7.1 Drugs that May Increase Risks Due to Fluid and Electrolyte Abnormalities
Use caution when prescribing PEG-3350 and Electrolytes for Oral Solution for patients with conditions and/or who are using medications that increase the risk for fluid and electrolyte disturbances or may increase the risk of renal impairment, seizure, arrhythmias, and prolonged QT in the setting of fluid and electrolyte abnormalities [see Warnings and Precautions ( 5.1, 5.2, 5.3, 5.4)] . Consider additional patient evaluations as appropriate.
7.2 Potential for Reduced Drug Absorption
PEG-3350 and Electrolytes for Oral Solution can reduce the absorption of other administered drugs. Administer oral medications within one hour before the start of administration of PEG-3350 and Electrolytes for Oral Solution [see Dosage and Administration ( 2.1)] .
7.3 Stimulant Laxatives
Concurrent use of stimulant laxatives and PEG-3350 and Electrolytes for Oral Solution may increase the risk of mucosal ulceration or ischemic colitis. Avoid use of stimulant laxatives (e.g., bisacodyl, sodium picosulfate) while taking PEG-3350 and Electrolytes for Oral Solution [see Warnings and Precautions ( 5.5)] .
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Animal reproduction studies have not been conducted with PEG-3350 and Electrolytes for Oral Solution. It is also not known whether PEG-3350 and Electrolytes for Oral Solution can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. PEG-3350 and Electrolytes for Oral Solution should be given to a pregnant woman only if clearly needed.
8.3 Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when PEG-3350 and Electrolytes for Oral Solution is administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness of PEG-3350 and Electrolytes for Oral Solution in pediatric patients have not been established.
8.5 Geriatric Use
Clinical studies of PEG-3350 and Electrolytes for Oral Solution did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
8.6 Renal Impairment
Use PEG-3350 and Electrolytes for Oral Solution with caution in patients with renal impairment or patients taking concomitant medications that may affect renal function [see Drug Interactions ( 7.1)] . These patients may be at risk for renal injury. Advise these patients of the importance of adequate hydration before, during and after use of PEG-3350 and Electrolytes for Oral Solution and consider performing baseline and post-colonoscopy laboratory tests (electrolytes, creatinine, and BUN) in these patients [see Warnings and Precautions ( 5.4)] .
-
11 DESCRIPTION
PEG-3350 and Electrolytes for Oral Solution is a combination of polyethylene glycol 3350, an osmotic laxative, and electrolytes (sodium sulfate, sodium chloride, sodium bicarbonate and potassium chloride) for oral solution supplied in a 4 liter disposable jug containing 236 g polyethylene glycol 3350, 22.74 g sodium sulfate (anhydrous), 6.74 g sodium bicarbonate, 5.86 g sodium chloride, and 2.97 g potassium chloride as a white powder.
Polyethylene Glycol 3350, USP
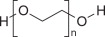
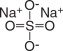
Sodium Sulfate, USP
The chemical name is Na 2SO 4. The average Molecular Weight is 142.04. The structural formula is:
Sodium Bicarbonate, USP
The chemical name is NaHCO 3. The average Molecular Weight is 84.01. The structural formula is:

Sodium Chloride, USP
The chemical name is NaCl. The average Molecular Weight: 58.44. The structural formula is:
Na + Cl -
Potassium Chloride, USP
The chemical name is KCl. The average Molecular Weight: 74.55. The structural formula is:
K-Cl
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The primary mode of action is thought to be through the osmotic effect of polyethylene glycol 3350 which causes water to be retained in the colon and produces a watery stool.
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-Approved Patient Labeling (Medication Guide and Instructions for Use).
Instruct patients:
- To reconstitute PEG-3350 and Electrolytes for Oral Solution with water prior to ingestion.
- Not to take other laxatives while they are taking PEG-3350 and Electrolytes for Oral Solution.
- Not to take oral medications within 1 hour before the start or during the administration of PEG-3350 and Electrolytes for Oral Solution.
- To take only clear liquids but avoid red and purple liquids.
- To consume water or other clear liquids during the bowel preparation and after completion of the bowel preparation up until 2 hours before the time of the colonoscopy.
- To follow the directions in the Instructions for Use on how to prepare and administer the product.
- If they experience severe bloating, distention or abdominal pain, to slow or temporarily discontinue drinking the solution and to contact their healthcare provider.
- To contact their healthcare provider if they develop signs and symptoms of dehydration or if they experience altered consciousness or seizures [see Warnings and Precautions ( 5.1, 5.2, 5.3, 5.4)] .
- To discontinue administration of the solution and contact their healthcare provider if they develop symptoms of a hypersensitivity reaction [see Warnings and Precautions ( 5.8)] .
Distributed by Affordable Pharmaceuticals, LLC, Braintree, MA 02185
-
MEDICATION GUIDE
Medication Guide
PEG-3350 and Electrolytes for Oral Solution
(PEG-3350 and i-lek-truh-lahyts for oral solution)Read this Medication Guide before you start taking PEG-3350 and Electrolytes for Oral Solution. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment. What is the most important information I should know about PEG-3350 and Electrolytes for Oral Solution?
PEG-3350 and Electrolytes for Oral Solution and other osmotic bowel preparations can cause serious side effects, including:
Serious loss of body fluid (dehydration) and changes in blood salts (electrolytes) in your blood.
These changes can cause:- abnormal heartbeats (arrhythmias) that can cause death.
- seizures. This can happen even if you have never had a seizure.
- kidney problems.
Your chance of having fluid loss and changes in body salts with PEG-3350 and Electrolytes for Oral Solution is higher if you:
- have heart problems.
- have kidney problems.
- take water pills or non-steroidal anti-inflammatory drugs (NSAIDS).
- vomiting that prevents you from keeping down the solution.
- dizziness.
- urinating less often than normal.
- headache.
What is PEG-3350 and Electrolytes for Oral Solution?
PEG-3350 and Electrolytes for Oral Solution is a prescription medicine used by adults to clean the colon before a colonoscopy or barium enema X-ray examination. PEG-3350 and Electrolytes for Oral Solution cleans your colon by causing you to have diarrhea (loose stools). Cleaning your colon helps your healthcare provider see the inside of your colon more clearly during your colonoscopy.
It is not known if PEG-3350 and Electrolytes for Oral Solution is safe and effective in children.
Who should not take PEG-3350 and Electrolytes for Oral Solution?
Do not takePEG-3350 and Electrolytes for Oral Solution if your healthcare provider has told you that you have:
- a blockage in your bowel (obstruction).
- an opening in the wall of your stomach or intestine (bowel perforation).
- a very dilated intestine (toxic megacolon).
- problems with food and fluid emptying from your stomach (gastric retention).
- a problem with food moving too slowly through your intestines (ileus).
- an allergy to any of the ingredients in PEG-3350 and Electrolytes for Oral Solution. See the end of this Medication Guide for a complete list of ingredients in PEG-3350 and Electrolytes for Oral Solution.
What should I tell my healthcare provider before taking PEG-3350 and Electrolytes for Oral Solution?
Before you take PEG-3350 and Electrolytes for Oral Solution, tell your healthcare provider if you:- have heart problems.
- have stomach or bowel problems.
- have ulcerative colitis.
- have problems with swallowing or gastric reflux.
- have a history of seizures.
- are withdrawing from drinking alcohol.
- have a low blood salt (sodium) level.
- have kidney problems.
- have any other medical conditions.
- are pregnant. It is not known if PEG-3350 and Electrolytes for Oral Solution will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if PEG-3350 and Electrolytes for Oral Solution passes into your breast milk. You and your healthcare provider should decide if you will take PEG-3350 and Electrolytes for Oral Solution while breastfeeding.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
PEG-3350 and Electrolytes for Oral Solution may affect how other medicines work. Do not take medicines by mouth within 1 hour of starting PEG-3350 and Electrolytes for Oral Solution or after you start taking PEG-3350 and Electrolytes for Oral Solution.
Especially tell your healthcare provider if you take:
- medicines for blood pressure or heart problems.
- medicines for kidney problems.
- medicines for seizures.
- water pills (diuretics).
- non-steroidal anti-inflammatory medicines (NSAID) pain medicines.
- laxatives.
- starch-based thickeners. For patients who have trouble swallowing, do not mix PEG-3350 and Electrolytes for Oral Solution with starch-based thickeners.
How should I take PEG-3350 and Electrolytes for Oral Solution?
You must read, understand, and follow these instructions to take PEG-3350 and Electrolytes for Oral Solution the right way.
- Take PEG-3350 and Electrolytes for Oral Solution exactly as your healthcare provider tells you to take it
- See the “Instructions for Use” on the bottle label for instructions on how to mix, take or give PEG-3350 and Electrolytes for Oral Solution.
- Do not take undissolved PEG-3350 and Electrolytes for Oral Solution powder that has not been mixed with water (diluted). It may increase your risk of nausea, vomiting and fluid loss (dehydration).
- Do not take other laxatives while taking PEG-3350 and Electrolytes for Oral Solution.
- Drink reconstituted solution at a rate of 8 ounces (240 ml) every 10 minutes. Rapid drinking of each portion is better than drinking small amounts.
- Do not eat or drink anything colored red or purple.
- Do not eat solid foods at least 2 hours before taking PEG-3350 and Electrolytes for Oral Solution. You may eat a light breakfast 2 hours before taking PEG-3350 and Electrolytes for Oral Solution. For best results, do not consume solid food for 3 to 4 hours before drinking PEG-3350 and Electrolytes for Oral Solution.
- Drink only water and clear liquids:
○ the day before your colonoscopy
○ while taking PEG-3350 and Electrolytes for Oral Solution
○ after taking PEG-3350 and Electrolytes for Oral Solution until 2 hours before your colonoscopy.- Drink clear liquids before, during, and after you take PEG-3350 and Electrolytes for Oral Solution to avoid fluid loss (dehydrated). Examples of clear liquids are:
○ water ○ clear broth
○ clear fruit juices without pulp including ○ clear soda
apple, white grape, or white cranberry ○ gelatin (without added fruit or
○ strained limeade or lemonade topping)
○ coffee or tea (Do not use any dairy ○ popsicles without pieces of
or non-dairy creamer) fruit or fruit pulp
- Drink clear liquids before, during, and after you take PEG-3350 and Electrolytes for Oral Solution to avoid fluid loss (dehydrated). Examples of clear liquids are:
- You may experience some abdominal bloating and distention before the bowels start to move. If severe discomfort or distention occur, slow or temporarily stop (discontinue) drinking the solution and contact your healthcare provider.
- The first bowel movement should occur approximately one hour after you start drinking the solution.
- Continue drinking until the watery stool is clear and free of solid matter.
What are the possible side effects of PEG-3350 and Electrolytes for Oral Solution?
PEG-3350 and Electrolytes for Oral Solution can cause serious side effects, including:
- See Section “What is the most important information I should know about PEG-3350 and Electrolytes for Oral Solution?”
-
changes in certain blood tests. Your healthcare provider may do blood tests before and after you take PEG-3350 and Electrolytes for Oral Solution to check your blood for changes. Tell your healthcare provider if you have any symptoms of too much fluid loss, including:
vomiting stomach (abdominal) cramping
nausea headache
bloating urinate less than usual
dizziness trouble drinking clear liquid - ulcers of the bowel or bowel problems (ischemic colitis). Tell your healthcare provider right away if you have severe stomach-area (abdomen) pain or rectal bleeding.
The most common side effects of PEG-3350 and Electrolytes for Oral Solution include:
nausea chest x-ray that shows water in the
stomach (abdominal) fullness lungs (infiltrate) after vomiting or
bloating inhaling food or liquid (aspirate).
stomach (abdominal) cramps anal irritationvomiting esophageal bleeding
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of PEG-3350 and Electrolytes for Oral Solution. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store PEG-3350 and Electrolytes for Oral Solution?
- Store PEG-3350 and Electrolytes for Oral Solution in a sealed container at room temperature, between 59ºF to 86°F (15ºC to 30°C).
- Store mixed (reconstituted) solution of PEG-3350 and Electrolytes for Oral Solution at 36° to 46°F (2°C to 8°C). Do not freeze.
- Use mixed (reconstituted) solution of PEG-3350 and Electrolytes for Oral Solution within 48 hours.
- After 48 hours, throw away (discard) any mixed (reconstituted) solution of PEG-3350 and Electrolytes for Oral Solution that is not used.
Keep PEG-3350 and Electrolytes for Oral Solution and all medicines out of the reach of children.
General information about the safe and effective use of PEG-3350 and Electrolytes for Oral Solution.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use PEG-3350 and Electrolytes for Oral Solution for a condition for which it was not prescribed. Do not give PEG-3350 and Electrolytes for Oral Solution to other people, even if they are going to have the same procedure you are. It may harm them.
This Medication Guide summarizes important information about PEG-3350 and Electrolytes for Oral Solution. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information that is written for healthcare professionals.What are the ingredients in PEG-3350 and Electrolytes for Oral Solution?
PEG-3350 and Electrolytes for Oral Solution comes in a 4-liter jug with PEG-3350 and Electrolytes for Oral Solution powder.
Active ingredients:
Powder for solution: polyethylene glycol 3350, sodium sulfate (anhydrous), sodium bicarbonate, sodium chloride, and potassium chloride.Inactive Ingredients: Pineapple Flavored PEG-3350 and Electrolytes for Oral Solution only (natural and artificial pineapple flavor powder, maltodextrin, gum arabic, sodium saccharin, silicon dioxide)
Affordable Pharmaceuticals, LLC
Braintree, MA 02185, USA
For more information go to www.affordablepharma.com or call 1-800-514-5617.This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: 05/2021 - PEG-3350 AND ELECTROLYTES
-
INGREDIENTS AND APPEARANCE
PEG-3350 AND ELECTROLYTES
polyethylene glycol 3350, sodium sulfate anhydrous, sodium bicarbonate, sodium chloride, potassium chloride powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50090-6521(NDC:10572-100) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) (POLYETHYLENE GLYCOL 3350 - UNII:G2M7P15E5P) POLYETHYLENE GLYCOL 3350 236 g in 4 L SODIUM SULFATE ANHYDROUS (UNII: 36KCS0R750) (SODIUM CATION - UNII:LYR4M0NH37, SULFATE ION - UNII:7IS9N8KPMG) SODIUM SULFATE ANHYDROUS 22.74 g in 4 L SODIUM BICARBONATE (UNII: 8MDF5V39QO) (SODIUM CATION - UNII:LYR4M0NH37, BICARBONATE ION - UNII:HN1ZRA3Q20) SODIUM BICARBONATE 6.74 g in 4 L SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 5.86 g in 4 L POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152, CHLORIDE ION - UNII:Q32ZN48698) POTASSIUM CHLORIDE 2.97 g in 4 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50090-6521-0 4 L in 1 JUG; Type 0: Not a Combination Product 06/13/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA019011 07/01/2009 Labeler - A-S Medication Solutions (830016429) Establishment Name Address ID/FEI Business Operations A-S Medication Solutions 830016429 RELABEL(50090-6521)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
