Isopropyl Alcohol by Becton Dickinson and Company ISOPROPYL ALCOHOL swab
Isopropyl Alcohol by
Drug Labeling and Warnings
Isopropyl Alcohol by is a Otc medication manufactured, distributed, or labeled by Becton Dickinson and Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Use
- Warning:
- Directions
- Other Information
- Inactive Ingredient
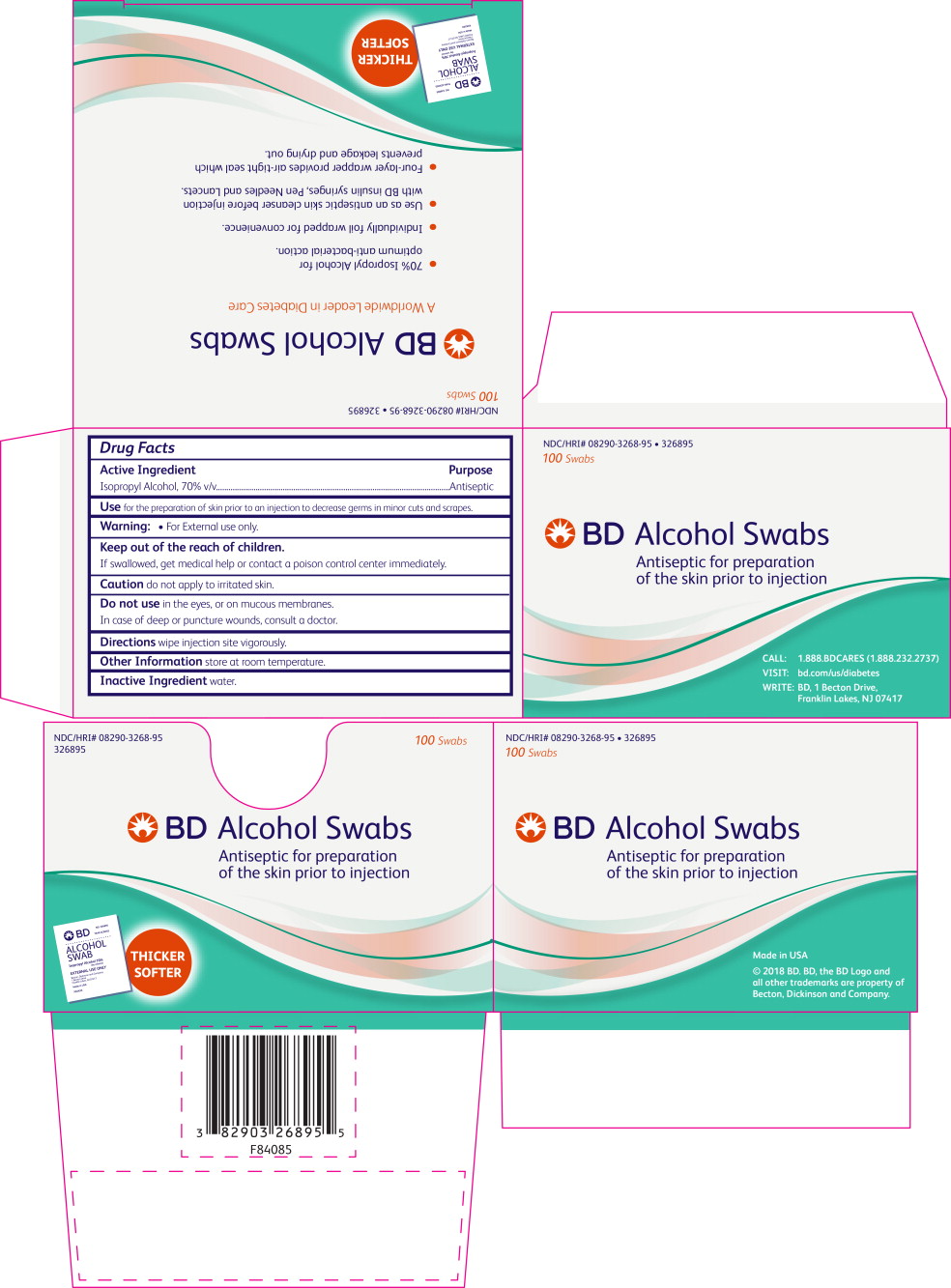
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ISOPROPYL ALCOHOL
isopropyl alcohol swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 8290-3268 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Isopropyl Alcohol (UNII: ND2M416302) (Isopropyl Alcohol - UNII:ND2M416302) Isopropyl Alcohol 0.75 g Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 8290-3268-95 12 in 1 CARTON 10/01/1993 1 100 in 1 BOX 1 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 10/01/1993 Labeler - Becton Dickinson and Company (001292192)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.