FEVERALL ADULTS- acetaminophen suppository
Feverall by
Drug Labeling and Warnings
Feverall by is a Otc medication manufactured, distributed, or labeled by Taro Pharmaceuticals U.S.A. Inc., G&W NC Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each rectal suppository)
- Purposes
- Uses
-
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if
- an adult or child 12 years and older takes more than 6 doses in 24 hours, which is the maximum daily amount
- taken with other drugs containing acetaminophen
- an adult takes 3 or more alcoholic drinks every day while using this product
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
Do not use
- in children under 12 years.
- if you are allergic to acetaminophen.
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 50 Suppository Carton
ADULTS'
ages 12 years & olderFeverAll®
ACETAMINOPHEN SUPPOSITORIESPain Reliever/Fever Reducer
NOT FOR RETAIL SALE
NDC: 51672-2117-4RECTAL
SUPPOSITORY**actual size
Doctor Recommended
50
Rectal
Suppositories650
mg
each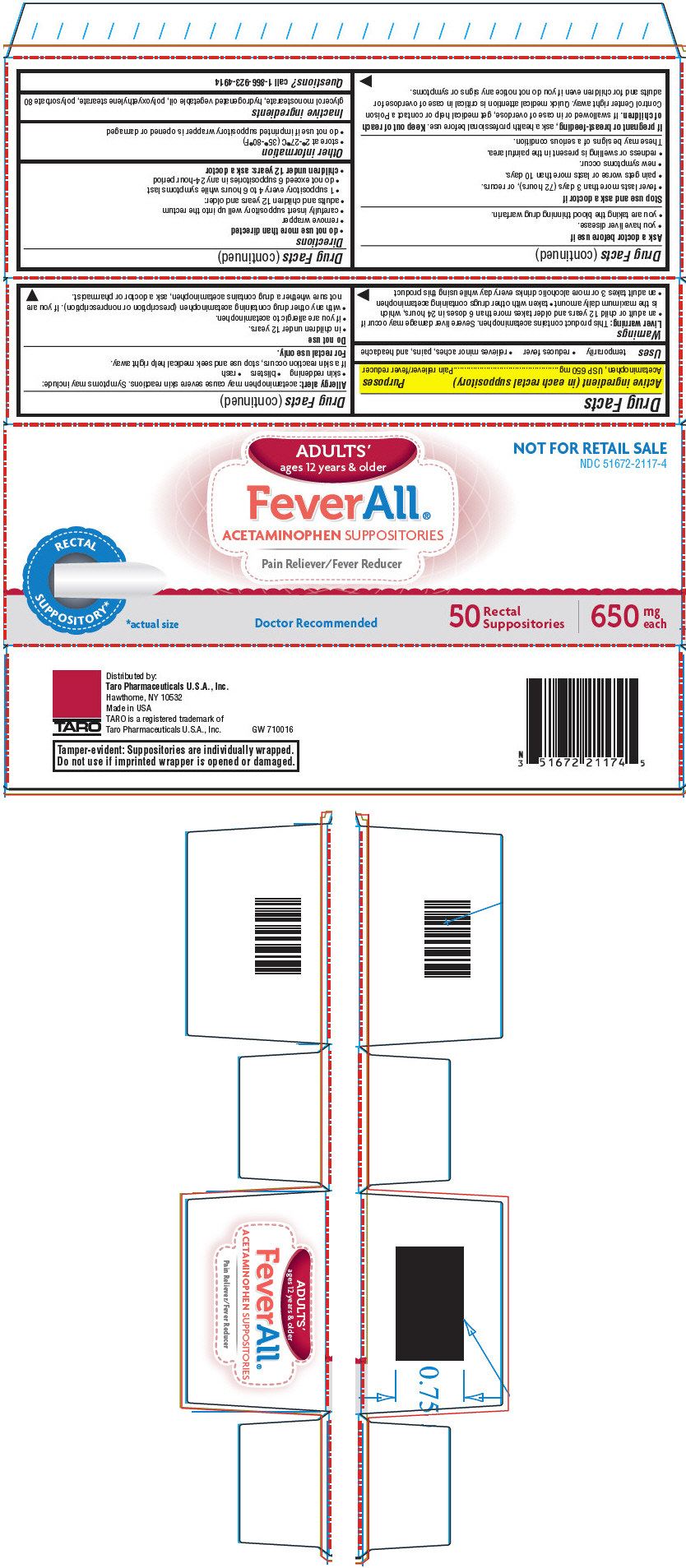
-
INGREDIENTS AND APPEARANCE
FEVERALL ADULTS
acetaminophen suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 51672-2117 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Acetaminophen (UNII: 362O9ITL9D) (Acetaminophen - UNII:362O9ITL9D) Acetaminophen 650 mg Inactive Ingredients Ingredient Name Strength glyceryl monostearate (UNII: 230OU9XXE4) hydrogenated palm kernel oil (UNII: FM8D1RE2VP) PEG-100 stearate (UNII: YD01N1999R) polysorbate 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51672-2117-4 50 in 1 CARTON 12/12/2013 1 NDC: 51672-2117-0 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018337 12/12/2013 Labeler - Taro Pharmaceuticals U.S.A. Inc. (145186370) Establishment Name Address ID/FEI Business Operations G&W NC Laboratories, Inc. 079419931 MANUFACTURE(51672-2117)
Trademark Results [Feverall]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 FEVERALL 85117574 3950872 Live/Registered |
TARO PHARMACEUTICAL INDUSTRIES, LTD. 2010-08-27 |
 FEVERALL 85039920 not registered Dead/Abandoned |
Actavis Mid Atlantic LLC 2010-05-17 |
 FEVERALL 74009487 1642430 Live/Registered |
UPSHER-SMITH LABORATORIES, INC. 1989-12-11 |
 FEVERALL 72144967 0756322 Dead/Expired |
MEDICS PHARMACEUTICAL CORPORATION 1962-05-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.