ACTHAR- repository corticotropin injection
Acthar by
Drug Labeling and Warnings
Acthar by is a Prescription medication manufactured, distributed, or labeled by Mallinckrodt ARD LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Acthar® Gel safely and effectively. See full prescribing information for Acthar Gel.
Acthar Gel (repository corticotropin injection) INJECTION, GEL for INTRAMUSCULAR | SUBCUTANEOUS use
Initial U.S. Approval: 1952INDICATIONS AND USAGE
- Acthar Gel is indicated as monotherapy for the treatment of infantile spasms in infants and children under 2 years of age. (1.1)
- Acthar Gel is indicated for the treatment of exacerbations of multiple sclerosis in adults. (1.2)
- Acthar Gel may be used for the following disorders and diseases: rheumatic; collagen; dermatologic; allergic states; ophthalmic; respiratory; and edematous state. (1.3 to 1.9)
DOSAGE AND ADMINISTRATION
- In the treatment of infantile spasms, the recommended dose is 150 U/m2 divided into twice daily intramuscular injections of 75 U/m2. After 2 weeks of treatment, dosing should be gradually tapered and discontinued over a 2-week period. (2.1)
- In the treatment of acute exacerbations of multiple sclerosis, daily intramuscular or subcutaneous doses of 80-120 units for 2-3 weeks may be administered. It may be necessary to taper the dose. (2.2)
- In the treatment of other disorders and diseases, dosing will need to be individualized depending on the disease under treatment and the medical condition of the patient. It may be necessary to taper the dose. (2.3)
DOSAGE FORMS AND STRENGTHS
- 5 mL multi-dose vial containing 80 USP units per mL. (3)
CONTRAINDICATIONS
- Acthar Gel should never be given intravenously.
- Acthar Gel is contraindicated in patients with scleroderma, osteoporosis, systemic fungal infections, ocular herpes simplex, recent surgery, history of or the presence of a peptic ulcer, congestive heart failure, uncontrolled hypertension, or sensitivity to proteins of porcine origin.
- Administration of live or live attenuated vaccines is contraindicated in patients receiving immunosuppressive doses of Acthar Gel.
- Acthar Gel is contraindicated in children under 2 years of age with suspected congenital infections. (4)
- Treatment of conditions listed within the INDICATIONS AND USAGE section is contraindicated when they are accompanied by primary adrenocortical insufficiency or adrenocortical hyperfunction. (4)
WARNINGS AND PRECAUTIONS
- Infections: Increased susceptibility to new infection and increased risk of exacerbation, dissemination or reactivation of latent infections. Signs and symptoms of infection may be masked. (5.1)
- Adrenal Insufficiency after Prolonged Therapy: Monitor for effects of hypothalamic-pituitary-axis suppression after stopping treatment. (5.2)
- Cushing's Syndrome: May occur after prolonged therapy. Monitor for signs and symptoms. (5.2)
- Elevated Blood Pressure, Salt and Water Retention and Hypokalemia: Monitor blood pressure and sodium and potassium levels. (5.3)
- Vaccination: Do not administer live or live attenuated vaccines to patients on immunosuppressive doses. (5.4)
- Masking of Symptoms of Other Underlying Disease/Disorders. Monitor patients for signs of other underlying disease/disorders that may be masked. (5.5)
- Gastrointestinal Perforation and Bleeding: There is a risk for gastric ulcers and bleeding. There is an increased risk of perforation in patients with certain GI disorders. Signs and symptoms may be masked. Monitor for signs of perforation and bleeding. (5.6)
- Behavioral and Mood Disturbances: May include euphoria, insomnia, mood swings, personality changes, severe depression and psychosis. Existing conditions may be aggravated. (5.7)
- Comorbid Diseases: Symptoms of diabetes and myasthenia gravis may be worsened with treatment. (5.8)
- Ophthalmic Effects: Monitor for cataracts, infections and glaucoma. (5.9)
- Immunogenicity Potential: Neutralizing antibodies with chronic administration may lead to a loss of endogenous ACTH activity. (5.10)
- Use in Patients with Hypothyroidism or Liver Cirrhosis: May result in an enhanced effect. (5.11)
- Negative Effects on Growth and Physical Development: Monitor pediatric patients on long term therapy. (5.12)
- Decrease in Bone Density: Monitor for osteoporosis in patients on long term therapy. (5.13)
- Use in Pregnancy: Embryocidal effect. Apprise women of potential harm to the fetus. (5.14)
ADVERSE REACTIONS
- Common adverse reactions for Acthar Gel are similar to those of corticosteroids and include fluid retention, alteration in glucose tolerance, elevation in blood pressure, behavioral and mood changes, increased appetite and weight gain. (6)
- Specific adverse reactions resulting from drug use in children under 2 years of age are increased risk of infections, hypertension, irritability, Cushingoid symptoms, cardiac hypertrophy and weight gain. (6.1.1)
To report SUSPECTED ADVERSE REACTIONS, contact Mallinckrodt at 1-800-778-7898 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Acthar Gel may accentuate the electrolyte loss associated with diuretic therapy. (7)
USE IN SPECIFIC POPULATIONS
- Pregnancy:Acthar Gel has been shown to have an embryocidal effect and should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. (8.1)
- Pediatric Use: Prolonged use of Acthar Gel in children may inhibit skeletal growth. If use is necessary, it should be given intermittently with careful observation. (5.12 and 8.4)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 3/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Infantile spasms
1.2 Multiple Sclerosis
1.3 Rheumatic Disorders
1.4 Collagen Diseases
1.5 Dermatologic Diseases
1.6 Allergic States
1.7 Ophthalmic Diseases
1.8 Respiratory Diseases
1.9 Edematous State
2 DOSAGE AND ADMINISTRATION
2.1 Specific Recommended Dosage Regimen for Infantile Spasms in Infants and Children Under 2 Years of Age
2.2 Recommended Dosage Regimen for the Treatment of Acute Exacerbations in Adults with Multiple Sclerosis
2.3 Recommended Dosage Regimen for Other Indications for Adults and Children Over 2 Years of Age
2.4 Preparation
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Infections
5.2 Cushing's Syndrome and Adrenal Insufficiency Upon Withdrawal
5.3 Elevated Blood Pressure, Salt and Water Retention and Hypokalemia
5.4 Vaccination
5.5 Masking Symptoms of Other Diseases
5.6 Gastrointestinal Perforation and Bleeding
5.7 Behavioral and Mood Disturbances
5.8 Comorbid Diseases
5.9 Ophthalmic Effects
5.10 Immunogenicity Potential
5.11 Use in Patients with Hypothyroidism or Liver Cirrhosis
5.12 Negative Effects on Growth and Physical Development
5.13 Decrease in Bone Density
5.14 Use in Pregnancy
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
6.3 Possible Additional Steroidogenic Effects
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED / STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Infantile spasms
Acthar Gel (repository corticotropin injection) is indicated as monotherapy for the treatment of infantile spasms in infants and children under 2 years of age.
1.2 Multiple Sclerosis
Acthar Gel (repository corticotropin injection) is indicated for the treatment of acute exacerbations of multiple sclerosis in adults. Controlled clinical trials have shown Acthar Gel to be effective in speeding the resolution of acute exacerbations of multiple sclerosis. However, there is no evidence that it affects the ultimate outcome or natural history of the disease.
1.3 Rheumatic Disorders
As adjunctive therapy for short-term administration (to tide the patient over an acute episode or exacerbation) in: Psoriatic arthritis; Rheumatoid arthritis, including juvenile rheumatoid arthritis (selected cases may require low-dose maintenance therapy), Ankylosing spondylitis.
1.4 Collagen Diseases
During an exacerbation or as maintenance therapy in selected cases of: systemic lupus erythematosus, systemic dermatomyositis (polymyositis).
-
2 DOSAGE AND ADMINISTRATION
2.1 Specific Recommended Dosage Regimen for Infantile Spasms in Infants and Children Under 2 Years of Age
In the treatment of infantile spasms, Acthar Gel must be administered intramuscularly. The recommended regimen is a daily dose of 150 U/m2 (divided into twice daily intramuscular injections of 75 U/m2) administered over a 2-week period. Dosing with Acthar Gel should then be gradually tapered over a 2-week period to avoid adrenal insufficiency. The following is one suggested tapering schedule: 30 U/m2 in the morning for 3 days; 15 U/m2 in the morning for 3 days; 10 U/m2 in the morning for 3 days; and 10 U/m2 every other morning for 6-days.
Acthar Gel is typically dosed based on body surface area (BSA). For calculation of body surface area, use the following formula
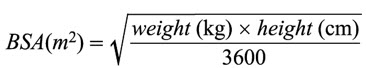
2.2 Recommended Dosage Regimen for the Treatment of Acute Exacerbations in Adults with Multiple Sclerosis
The recommended dose is daily intramuscular or subcutaneous doses of 80-120 units for 2-3 weeks for acute exacerbations.
Dosage should be individualized according to the medical condition of each patient. Frequency and dose of the drug should be determined by considering the severity of the disease and the initial response of the patient.
Although drug dependence does not occur, sudden withdrawal of Acthar Gel after prolonged use may lead to adrenal insufficiency or recurrent symptoms which make it difficult to stop the treatment. It may be necessary to taper the dose and increase the injection interval to gradually discontinue the medication.
2.3 Recommended Dosage Regimen for Other Indications for Adults and Children Over 2 Years of Age
Dosage should be individualized according to the disease under treatment and the general medical condition of each patient. Frequency and dose of the drug should be determined by considering severity of the disease and the initial response of the patient.
The usual dose of Acthar Gel is 40-80 units given intramuscularly or subcutaneously every 24-72 hours.
Although drug dependence does not occur, sudden withdrawal of Acthar Gel after prolonged use may lead to adrenal insufficiency or recurrent symptoms which make it difficult to stop the treatment. It may be necessary to taper the dose and increase the injection interval to gradually discontinue the medication.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Acthar Gel is contraindicated for intravenous administration.
Acthar Gel is contraindicated where congenital infections are suspected in infants.
Administration of live or live attenuated vaccines is contraindicated in patients receiving immunosuppressive doses of Acthar Gel.
Acthar Gel is contraindicated in patients with scleroderma, osteoporosis, systemic fungal infections, ocular herpes simplex, recent surgery, history of or the presence of a peptic ulcer, congestive heart failure, uncontrolled hypertension, primary adrenocortical insufficiency, adrenocortical hyperfunction or sensitivity to proteins of porcine origin.
-
5 WARNINGS AND PRECAUTIONS
The adverse effects of Acthar Gel are related primarily to its steroidogenic effects. Not all of the adverse events described below have been seen after treatment with Acthar Gel, but might be expected to occur [see Adverse Reactions (6.3)].
5.1 Infections
Acthar Gel may increase the risks related to infections with any pathogen, including viral, bacterial, fungal, protozoan or helminthic infections. Patients with latent tuberculosis or tuberculin reactivity should be observed closely, and if therapy is prolonged, chemoprophylaxis should be instituted.
5.2 Cushing's Syndrome and Adrenal Insufficiency Upon Withdrawal
Treatment with Acthar Gel can cause hypothalamic-pituitary-axis (HPA) suppression and Cushing's syndrome. These conditions should be monitored especially with chronic use.
Suppression of the HPA may occur following prolonged therapy with the potential for adrenal insufficiency after withdrawal of the medication. Patients should be monitored for signs of insufficiency such as weakness, hyperpigmentation, weight loss, hypotension and abdominal pain.
The symptoms of adrenal insufficiency in infants treated for infantile spasms can be difficult to identify. The symptoms are non-specific and may include anorexia, fatigue, lethargy, weakness, excessive weight loss, hypotension and abdominal pain. It is critical that parents and caregivers be made aware of the possibility of adrenal insufficiency when discontinuing Acthar Gel and should be instructed to observe for, and be able to recognize, these symptoms [see Patient Counseling Information (17)].
The recovery of the adrenal gland may take from days to months so patients should be protected from the stress (e.g. trauma or surgery) by the use of corticosteroids during the period of stress.
The adrenal insufficiency may be minimized in adults and infants by tapering of the dose when discontinuing treatment.
Signs or symptoms of Cushing's syndrome may occur during therapy but generally resolve after therapy is stopped. Patients should be monitored for these signs and symptoms such as deposition of adipose tissue in characteristics sites (e.g., moon face, truncal obesity), cutaneous striae, easy bruisability, decreased bone mineralization, weight gain, muscle weakness, hyperglycemia, and hypertension.
5.3 Elevated Blood Pressure, Salt and Water Retention and Hypokalemia
Acthar Gel can cause elevation of blood pressure, salt and water retention, and increased excretion of potassium and calcium. Dietary salt restriction and potassium supplementation may be necessary. Caution should be used in the treatment of patients with hypertension, congestive heart failure, or renal insufficiency.
5.4 Vaccination
Administration of live or live attenuated vaccines is contraindicated in patients receiving immunosuppressive doses of Acthar Gel. Killed or inactivated vaccines may be administered; however, the response to such vaccines can not be predicted. Other immunization procedures should be undertaken with caution in patients who are receiving Acthar Gel, especially when high doses are administered, because of the possible hazards of neurological complications and lack of antibody response.
5.5 Masking Symptoms of Other Diseases
Acthar Gel often acts by masking symptoms of other diseases/disorders without altering the course of the other disease/disorder. Patients should be monitored carefully during and for a period following discontinuation of therapy for signs of infection, abnormal cardiac function, hypertension, hyperglycemia, change in body weight and fecal blood loss.
5.6 Gastrointestinal Perforation and Bleeding
Acthar Gel can cause GI bleeding and gastric ulcer. There is also an increased risk for perforation in patients with certain gastrointestinal disorders. Signs of gastrointestinal perforation, such as peritoneal irritation, may be masked by the therapy. Use caution where there is the possibility of impending perforation, abscess or other pyogenic infections, diverticulitis, fresh intestinal anastomoses, and active or latent peptic ulcer.
5.7 Behavioral and Mood Disturbances
Use of Acthar Gel may be associated with central nervous system effects ranging from euphoria, insomnia, irritability (especially in infants), mood swings, personality changes, and severe depression, to frank psychotic manifestations. Also, existing emotional instability or psychotic tendencies may be aggravated.
5.8 Comorbid Diseases
Patients with a comorbid disease may have that disease worsened. Caution should be used when prescribing Acthar Gel in patients with diabetes and myasthenia gravis.
5.9 Ophthalmic Effects
Prolonged use of Acthar Gel may produce posterior subcapsular cataracts, glaucoma with possible damage to the optic nerves and may enhance the establishment of secondary ocular infections due to fungi and viruses.
5.10 Immunogenicity Potential
Acthar Gel is immunogenic. Limited available data suggest that a patient may develop antibodies to Acthar Gel after chronic administration and loss of endogenous ACTH and Acthar Gel activity. Prolonged administration of Acthar Gel may increase the risk of hypersensitivity reactions. Sensitivity to porcine protein should be considered before starting therapy and during the course of treatment should symptoms arise.
5.11 Use in Patients with Hypothyroidism or Liver Cirrhosis
There is an enhanced effect in patients with hypothyroidism and in those with cirrhosis of the liver.
5.12 Negative Effects on Growth and Physical Development
Long-term use of Acthar Gel may have negative effects on growth and physical development in children. Changes in appetite are seen with Acthar Gel therapy, with the effects becoming more frequent as the dose or treatment period increases. These effects are reversible once Acthar Gel therapy is stopped. Growth and physical development of pediatric patients on prolonged therapy should be carefully monitored.
5.13 Decrease in Bone Density
Decrease in bone formation and an increase in bone resorption both through an effect on calcium regulation (i.e. decreasing absorption and increasing excretion) and inhibition of osteoblast function may occur. These, together with a decrease in the protein matrix of the bone (secondary to an increase in protein catabolism) and reduced sex hormone production, may lead to inhibition of bone growth in children and adolescents and to the development of osteoporosis at any age. Special consideration should be given to patients at increased risk of osteoporosis (i.e., postmenopausal women) before initiating therapy, and bone density should be monitored in patients on long term therapy.
5.14 Use in Pregnancy
Acthar Gel has been shown to have an embryocidal effect. Apprise women of potential harm to the fetus [see Use in Specific Populations (8.1)].
-
6 ADVERSE REACTIONS
Please refer to Adverse Reactions in Infants and Children Under 2 Years of Age (Section 6.1.1) for consideration when treating patients with Infantile Spasms. The adverse reactions presented in Section 6.2 are primarily provided for consideration in use in adults and in children over 2 years of age, but these adverse reactions should also be considered when treating infants and children under 2 years of age.
Acthar Gel causes the release of endogenous cortisol from the adrenal gland. Therefore all the adverse effects known to occur with elevated cortisol may occur with Acthar Gel administration as well. Common adverse reactions include fluid retention, alteration in glucose tolerance, elevation in blood pressure, behavioral and mood changes, increased appetite and weight gain.
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug, and may not reflect the rates observed in practice.
6.1.1 Adverse Reactions in Infants and Children Under 2 Years of Age
While the types of adverse reactions seen in infants and children under age 2 treated for infantile spasms are similar to those seen in older patients, their frequency and severity may be different due to the very young age of the infant, the underlying disorder, the duration of therapy and the dosage regimen. Below is a summary of adverse reactions specifically tabulated from source data derived from retrospective chart reviews and clinical trials in children under 2 years of age treated for infantile spasms. The number of patients in controlled trials at the recommended dose was too few to provide meaningful incidence rates or to permit a meaningful comparison to the control groups.
TABLE: Incidence (%) of Treatment Emergent Adverse Events Occurring in ≥ 2% of Acthar Gel (repository corticotropin injection) Infants and Children under 2 years of Age System Organ Class Recommended
75 U/m2 bid
n=122, (%)150 U/m2 qd
n=37 (%)- * Specific infections that occurred at ≥ 2% were candidiasis, otitis media, pneumonia and upper respiratory tract infections.
- † In the treatment of Infantile Spasms, other types of seizures/convulsions may occur because some patients with infantile spasms progress to other forms of seizures (for example, Lennox-Gastaut Syndrome). Additionally, the spasms sometimes mask other seizures and once the spasms resolve after treatment, the other seizures may become visible.
Cardiac disorders Cardiac Hypertrophy 3 0 Endocrine disorders Cushingoid 3 22 Gastrointestinal disorders Constipation 0 5 Diarrhea 3 14 Vomiting 3 5 General disorders and administration site conditions Irritability 7 19 Pyrexia 5 8 Infections and infestations Infection* 20 46 Investigations Weight gain 1 3 Metabolism and nutrition disorders Increased appetite 0 5 Decreased appetite 3 3 Nervous system disorders Convulsion† 12 3 Respiratory, thoracic and mediastinal disorders Nasal Congestion 1 5 Skin and subcutaneous tissue disorders Acne 0 14 Rash 0 8 Vascular disorders Hypertension 11 19 These adverse reactions may also be seen in adults and children over 2 years of age when treated for other purposes and with different doses and regimens.
6.2 Postmarketing Experience
The following adverse reactions associated with the use of Acthar Gel have been identified from postmarketing experience with Acthar Gel. Only adverse events that are not listed above as adverse events reported from retrospective chart reviews and non-sponsor conducted clinical trials and those not discussed elsewhere in labeling, are listed in this section. Because the adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency or establish a causal relationship to use with Acthar Gel. Events are categorized by system organ class. Unless otherwise noted these adverse events have been reported in infants, children and adults.
6.2.1 Allergic Reactions
Allergic responses have presented as dizziness, nausea and shock (adults only).
6.3 Possible Additional Steroidogenic Effects
Based on steroidogenic effects of Acthar Gel certain adverse events may be expected due to the pharmacological effects of corticosteroids. The adverse events that may occur but have not been reported for Acthar Gel are:
6.3.1 Dermatologic
Impaired wound healing, abscess, petechiae and ecchymoses, and suppression of skin test reactions.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.3 Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Acthar Gel, when treating a nursing mother, a decision should be made whether to discontinue nursing or to discontinue the drug, considering the risk and benefit to the mother.
8.4 Pediatric Use
Acthar Gel is indicated as monotherapy for the treatment of infantile spasms in infants and children less than 2 years of age. Both serious and other adverse reactions in this population are discussed in Warnings and Adverse Reactions in Infants and Children Under 2 Years of Age [see Sections 5 and 6.1.1].
The efficacy of Acthar Gel for the treatment of infantile spasms in infants and children less than 2 years of age was evaluated in a randomized, single blinded (video EEG interpreter blinded) clinical trial and an additional active control supportive trial [see Clinical Studies (14)]. A responding patient was defined as having both complete cessation of spasms and elimination of hypsarrhythmia.
Safety in the pediatric population for infantile spasms was evaluated by retrospective chart reviews and data from non-sponsor conducted clinical trials [see Adverse Reactions (6.1.1)]. While the types of adverse reactions seen in infants and children under 2 years of age treated for infantile spasms are similar to those seen in older patients, their frequency and severity may be different due to the very young age of the infant, the underlying disorder, the duration of therapy and the dosage regimen. Effects on growth are of particular concern [see Warnings and Precautions (5.12)]. Serious adverse reactions observed in adults may also occur in children [see Warnings and Precautions (5)].
-
10 OVERDOSAGE
While chronic exposure to Acthar Gel at high doses can be associated with a variety of potential serious adverse effects, it is not expected that a single high dose, or even several large doses, has the potential for serious adverse effects compared to a standard dose. There have been no reports of death or acute overdose symptoms from Acthar Gel in clinical studies or in the published literature.
The intramuscular route of administration makes it unlikely that an inadvertent acute overdose will occur. The typical daily dose of Acthar Gel to treat an infant that has a BSA of 0.4 m2 would be 60 U/day. Using the 1-cc syringe supplied with Acthar Gel, the maximum amount that can be injected is 80 U/injection, which is a well-tolerated single dose.
-
11 DESCRIPTION
Acthar Gel is a naturally sourced complex mixture of adrenocorticotropic hormone analogs and other pituitary peptides. The Acthar Gel manufacturing process converts the initial porcine pituitary extract with low ACTH content into a mixture having modified porcine ACTH and other related peptide analogs solubilized in gelatin. A major component in the formulated complex mixture is N-25 deamidated porcine ACTH (1-39).
Acthar Gel is supplied as a sterile preparation in 16% gelatin to provide a prolonged release after intramuscular or subcutaneous injection. Acthar Gel also contains 0.5% phenol, not more than 0.1% cysteine (added), sodium hydroxide and/or acetic acid to adjust pH and water for injection.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of Acthar Gel in the treatment of infantile spasms is unknown.
Acthar Gel and endogenous ACTH stimulate the adrenal cortex to secrete cortisol, corticosterone, aldosterone, and a number of weakly androgenic substances. Prolonged administration of large doses of Acthar Gel induces hyperplasia and hypertrophy of the adrenal cortex and continuous high output of cortisol, corticosterone and weak androgens. The release of endogenous ACTH is under the influence of the nervous system via the regulatory hormone released from the hypothalamus and by a negative corticosteroid feedback mechanism. Elevated plasma cortisol suppresses ACTH release.
Acthar Gel is also reported to bind to melanocortin receptors.
The trophic effects of endogenous ACTH and Acthar Gel on the adrenal cortex are not well understood beyond the fact that they appear to be mediated by cyclic AMP.
ACTH rapidly disappears from the circulation following its intravenous administration; in people, the plasma half-life is about 15 minutes. The pharmacokinetics of Acthar Gel have not been adequately characterized.
The maximal effects of a trophic hormone on a target organ are achieved when optimal amounts of hormone are acting continuously. Thus, a fixed dose of Acthar Gel will demonstrate a linear increase in adrenocortical secretion with increasing duration for the infusion.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Adequate and well-controlled studies have not been done in animals. Human use has not been associated with an increase in malignant disease [see Warnings and Precautions (5.14) and Use in Specific Populations (8.1)].
-
14 CLINICAL STUDIES
The effectiveness of Acthar Gel as a treatment for infantile spasms was demonstrated in a single blinded (video EEG interpreter blinded) clinical trial in which patients were randomized to receive either a 2 week course of treatment with Acthar Gel (75 U/m2 intramuscular twice daily) or prednisone (1 mg/kg by mouth twice daily). The primary outcome was a comparison of the number of patients in each group who were treatment responders, defined as a patient having complete suppression of both clinical spasms and hypsarrhythmia on a full sleep cycle video EEG performed 2 weeks following treatment initiation, rated by an investigator blinded to treatment. Thirteen of 15 patients (86.7%) responded to Acthar Gel as compared to 4 of 14 patients (28.6%) given prednisone (p<0.002). The 2-week treatment was followed by a 2-week period of taper. Nonresponders to the prednisone treatment were eligible to receive Acthar Gel treatment. Seven of 8 patients (87.5%) responded to Acthar Gel after not responding to prednisone. Similarly, the 2 nonresponder patients from the Acthar Gel treatment were eligible to receive treatment with prednisone. One of the 2 patients (50%) responded to the prednisone treatment after not responding to Acthar Gel.
A supportive single-blind, randomized clinical trial comparing high-dose, long-duration treatment (150 U/m2 once daily for 3 weeks, n=30) of Acthar Gel with low-dose, short-duration treatment (20 U once daily for 2 weeks, n=29) for the treatment of infantile spasms was also evaluated in infants and children less than 2 years of age. Nonresponders (defined as in the previously described study) in the low-dose group received a dose escalation at 2 weeks to 30 U once daily. Nominal statistical superiority of the high dose treatment, as compared to the low dose treatment, was observed for cessation of spasms but not for the resolution of hypsarrhythmia.
-
16 HOW SUPPLIED / STORAGE AND HANDLING
Acthar Gel (repository corticotropin injection) is supplied as 5 mL multi-dose vial (63004-8710-1) containing 80 USP Units per mL. Acthar Gel (repository corticotropin injection) should be warmed to room temperature before using. Do not over pressurize the vial prior to withdrawing the product.
-
17 PATIENT COUNSELING INFORMATION
Caretakers of patients with infantile spasms should be informed of the availability of a Medication Guide, and they should be instructed to read the Medication Guide prior to administering Acthar Gel. Patients should be instructed to take Acthar Gel only as prescribed. They should not stop treatment suddenly unless instructed by their physician to do so.
Patients, their caregivers and families should be advised as to the importance of the need for careful monitoring while on and during titration from Acthar Gel treatment and the importance of not missing scheduled doctor's appointments.
Patients, their caregivers and families should be advised that if the patient develops an infection or fever they should contact their physician. They should be educated that a fever may not necessarily be present during infection. The patient should also try to limit contact with other people with infections to minimize the risk of infection while taking Acthar Gel [see Warnings and Precautions (5.1) and Adverse Reactions (6.1.1)].
Patients, their caregivers and families should be advised that if the patient experiences an increase in blood pressure they should contact their physician [see Warnings and Precautions (5.3) and Adverse Reactions (6.1.1)].
Patients, their caregivers and families should be advised that if the patient or the caregiver notices blood or a change in color of the patient's stool they should contact their physician [see Warnings and Precautions (5.6)].
Caregivers and families of infants and children treated with Acthar Gel should be informed that the patient may show signs of irritability and sleep disturbances. These effects are reversible once Acthar Gel therapy is stopped [see Warnings and Precautions (5.7) and Adverse Reactions (6.1.1)].
Patients, their caregivers and families should be advised that changes in appetite, most often leading to weight gain, are seen with Acthar Gel therapy, becoming more frequent as the dose or treatment period increases. These effects are reversible once Acthar Gel therapy is stopped [see Warnings and Precautions (5.12) and Adverse Reactions (6.1.1)].
Patients, their caregivers and families should be advised that the patient may be monitored for signs of adrenal insufficiency such as weakness, fatigue, lethargy, anorexia, weight loss, hypotension, abdominal pain or hyperpigmentation (adults only) after treatment has stopped. Since the recovery of the adrenal gland varies from days to months, patients may need to be protected from the stress of trauma or surgery by the use of corticosteroids during the period of stress [see Warnings and Precautions (5.2)].
Patients should be advised not to be vaccinated with live or live attenuated vaccines during treatment with Acthar Gel. Additionally, other immunization procedures in patients or in family members who will be in contact with the patient should be undertaken with caution while the patient is taking Acthar Gel [see Warnings and Precautions (5.4)].
Patients, their caregivers and families should be advised that prolonged use of Acthar Gel in children may result in Cushing's syndrome and associated adverse reactions, may inhibit skeletal growth, and may cause osteoporosis and decreased bone density. If prolonged use is necessary, Acthar Gel should be given intermittently along with careful observation [see Warnings and Precautions (5.2), (5.12), and (5.13) and Adverse Reactions (6.1.1)].
Patients, their caregivers and families should be informed that Acthar Gel may mask symptoms of other diseases/disorders without altering the course of the other disease/disorder. The patient will need to be monitored carefully during and for a period following discontinuation of therapy for signs of infection, abnormal cardiac function, hypertension, hyperglycemia, change in body weight, and fecal blood loss [see Warnings and Precautions (5.5)].
In the treatment of Infantile Spasms, other types of seizures may occur because some patients with infantile spasms progress to other forms of seizures (for example, Lennox-Gastaut Syndrome). Additionally the spasms sometimes mask other seizures and once the spasms resolve after treatment with Acthar Gel, the other seizures may become visible. Parents and caregivers should inform their physician of any new onset of seizures so that appropriate management can then be instituted [see Adverse Reactions (6.1.1)].
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
Acthar® Gel (AK-thar jel)
(repository corticotropin Injection)This Medication Guide provides information only about the use of Acthar Gel for the treatment of Infantile Spasms. If your doctor prescribes Acthar Gel for you or your child for any other reason, talk to your doctor for information about how this medicine is used to treat your medical condition.
Read this Medication Guide before your child receives Acthar Gel and each time you refill your child's prescription. There may be new information. This Medication Guide does not take the place of talking with your doctor about your child's medical condition or treatment.
What is the most important information I should know about Acthar GEL?
Acthar Gel can cause serious side effects including:
- 1.
Increased risk of infections.
Acthar Gel is a medicine that can affect your child's immune system. When your child is taking Acthar Gel, it can lower the ability of your child's immune system to fight infections.
Acthar Gel may:
- make your child more likely to get new infections
- worsen an infection that your child already has
- cause an inactive infection to become active, such as tuberculosis (TB)
- an infection or signs of an infection, such as:
- fever
- cough
- vomiting
- diarrhea
- other signs of flu or illness
- a family member with an infection or signs of an infection
- stay away from people who are sick or who have infections
- tell your doctor right away if your child has any sign of infection such as:
- fever (but your child may not have a fever with an infection)
- cough
- vomiting
- diarrhea or
- other signs of illness or flu and
- any open cuts or sores on his or her body
- 2. Effects on the adrenal gland after stopping Acthar Gel.
When your child stops taking Acthar Gel, his or her body may not produce enough of a hormone called cortisol on its own (adrenal insufficiency). Your child may need to take steroid medicine to protect the body until the adrenal gland recovers and is working well again, especially to protect the body if they have surgery or trauma. Do not stop giving your child injections of Acthar Gel without talking to your doctor first.
Your doctor will tell you when and how to slowly stop giving the injections to avoid serious side effects.
While slowly stopping your child's injections of Acthar Gel or after you stop giving the injections, call your doctor right away if your child has any of the following:
- appears weak
- loses weight or has a decrease in appetite
- appears tired or lacking energy
- appears pale
- has stomach pain
- appears sick or is with a fever
- 3. Effects on the adrenal gland while taking Acthar Gel.
When your child is taking Acthar Gel, his or her adrenal gland may produce too much cortisol. This can cause symptoms of Cushing's syndrome. Cushing's syndrome is more common in children who take Acthar Gel for a long time.
Symptoms of Cushing's syndrome include:
- increased upper body fat around the neck, but not the arms and legs
- weight gain
- rounded or "moon" face
- thin skin, easy bruising, and stretch marks on thighs, belly and trunk
- slowed growth rates in children
- weak bones (osteoporosis)
- increased blood pressure. Your doctor may check your child's blood pressure during treatment. If your child's blood pressure increases, your doctor may talk with you about possible treatment choices.
- too much water in the body (water retention), increased amount of body salts, and low potassium in the blood. Acthar Gel may cause your child to have an increased amount of body salts and water that stays in the body, and may lower the amount of potassium in your child's blood. Follow your doctor's instructions about if you need to decrease your child's salt intake or if you need to feed your child foods high in potassium.
- 4. Your child should not receive certain vaccines during treatment with Acthar Gel.
Your child may receive killed or inactivated vaccines while receiving Acthar Gel. Before your child receives any vaccines, talk to your doctor about which vaccines are safe for your child. Certain vaccines could cause your child to have serious side effects, or the vaccine may not be effective.
- 5. Hiding (masking) symptoms of other conditions or diseases.
It may be more difficult for your doctor to diagnose other conditions or diseases in your child during treatment with Acthar Gel. During treatment and after treatment ends, tell your doctor if your child has:
- any signs or symptoms of infection. See number 1 of this section in the Medication Guide.
- changes in body weight
- bloody or black tarry stool
- vomiting
- stomach pain
- excessive tiredness
- increased thirst
- fast heart rate
- difficulty breathing
- 6. Stomach and intestinal problems. Acthar Gel may cause bleeding of the stomach or intestine.
Your child has an increased risk for bleeding from the stomach or having a stomach ulcer. Tell your doctor if your child has any pain in the stomach area (abdominal pain), vomits blood, or has bloody or black stools.
- 7. Changes in mood and behavior.
During treatment with Acthar Gel your child may be irritable, have rapid changes in his or her mood, be depressed, have other changes in his or her behavior, or have trouble sleeping.
Tell your doctor if your child has any of the side effects or symptoms listed above.
What is Acthar GEL?
Acthar Gel is a prescription medicine that is used to treat infantile spasms in infants and children under 2 years of age.
What should I tell my doctor before my child takes Acthar GEL?
Before your child takes Acthar Gel, read the section above "What is the most important information I should know about Acthar Gel?" and tell your doctor if your child has:
- an infection
- Diabetes
- heart problems
- kidney problems
- stomach or intestinal problems
- thyroid problems
- liver problems
- neuromuscular problems
- convulsions or seizures
- had exposure to someone with Tuberculosis (TB)
- a previous allergic reaction such as hives, itching or trouble breathing, to Acthar Gel or pork products
- had recent surgery
- had a recent vaccination or is scheduled to receive a vaccination
- a family member who is receiving vaccinations
Tell your doctor about all the medicines your child takes, including prescription and non-prescription medicines, vitamins and herbal supplements. Do not start giving a new medicine to your child without first speaking to your doctor.
How should I give Acthar Gel to my child?
Acthar Gel is given as an injection into the muscle. Do not inject it under the skin, into a vein, or give it to your child by mouth.
- Inject Acthar Gel exactly as your doctor tells you. Your doctor will tell you where to give the injection, how much to give, how often and when to give it to your child.
- Do not use Acthar Gel until your doctor has taught you how to give the injection to your child.
- To give Acthar Gel:
- Take the bottle from the refrigerator. Do not open the bottle or pry the cap (rubber stopper) off.
- Warm the contents by rolling the bottle between your hands for a few minutes.
- Wash your hands.
- Prepare the skin where you are going to give the injection by wiping it with a new sterile alcohol wipe. Before giving the injection, look at the site prepared for the injection and make sure that it no longer looks wet. A wet site can cause burning.
- Wipe the top of the vial rubber stopper with a new sterile alcohol wipe.
- Use a new sterile needle and syringe to draw up the amount of Acthar Gel the doctor has told you to use.
- Give the injection the way the doctor has instructed you.
- Return the bottle to the refrigerator as soon as possible.
Keep all of your child's follow-up appointments with your doctor
- It is important for you to tell your doctor if your child's spasms continue or change in any way during treatment or after treatment has stopped so that they can monitor your child's progress.
Infantile Spasms sometimes hides (masks) other seizures your child or infant may have. Once treated with Acthar Gel, the Infantile Spasms symptoms may disappear. This may allow the other seizures to become visible for the first time. Tell your child's doctor right away if you see a change in your child's seizures/spasms.
What are the possible side effects of Acthar Gel?
Acthar Gel can cause serious side effects.
- See "What is the most important information I should know about Acthar Gel."
- Acthar Gel may make certain other medical conditions worse, such as diabetes (may increase blood sugar).
- Eye problems. Your child can get cataracts, increased pressure in the eye (glaucoma), and possible damage to the optic nerve if treated with Acthar Gel for a long time.
-
Allergic reactions to Acthar Gel. Your child may have an allergic reaction to Acthar Gel. Allergic reactions may not happen until your child has received several injections of Acthar Gel. Tell your doctor right away if your child has any of the following signs of an allergic reaction:
- skin rash
- swelling of the face, tongue, lips, or throat
- trouble breathing
- Changes in growth and physical development. Acthar Gel may affect your child's growth and physical development and may weaken his or her bones. This is more likely to happen with long term use of Acthar Gel.
- Enlarged heart. Acthar Gel may cause an increase in the size of your child's heart. This is more likely to happen with long term use of Acthar Gel but usually goes away after Acthar Gel is stopped.
Common side effects of Acthar Gel may include:
- infections
- increased blood pressure
- irritability and changes in behavior
- changes in appetite and weight
- diarrhea
- vomiting
These are not all the possible side effects of Acthar Gel. Tell your doctor if your child has any side effect that bothers them or does not go away. For more information, ask your child's doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Acthar GEL?
- Store vials of Acthar Gel in the refrigerator between 36°F to 46°F (2°C to 8°C).
Throw away any vials after the expiration date printed on the label.
Keep Acthar Gel and all other medicines out of the reach of children
General information About Acthar Gel
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Acthar Gel for a condition for which it has not been prescribed. Do not give Acthar Gel to other people, even if they have the same symptoms. It may harm them.
This Medication Guide summarizes the most important information about Acthar Gel. If you would like more information, talk with your child's doctor. You can ask your child's doctor or pharmacist for information about Acthar Gel that is written for healthcare professionals. For more information, go to www.acthar.com or call 1-800-778-7898.
What are the ingredients in Acthar Gel?
Active ingredient: Corticotropin
Inactive ingredients: gelatin, phenol, cysteine, sodium hydroxide and/or acetic acid to adjust pH, and water for injectionManufactured for:
Mallinckrodt ARD LLC.
Bedminster, NJ 07921 USAPL122
Rev 3/2019This Medication Guide has been approved by the U.S. Food and Drug Administration.
Mallinckrodt, the "M" brand mark, the Mallinckrodt Pharmaceuticals logo and other brands are trademarks of a Mallinckrodt company.
© 2019 Mallinckrodt.
- 1.
Increased risk of infections.
-
PRINCIPAL DISPLAY PANEL - 5 mL Vial Carton
NDC: 63004-8710-1
Acthar®
Gel(repository corticotropin
injection)400 USP units/5 mL
(80 USP units/mL)For intramuscular or
subcutaneous use.5 mL
multiple-dose vialDispense the enclosed
Medication Guide to each
Infantile Spasm patient.Rx only
-
INGREDIENTS AND APPEARANCE
ACTHAR
repository corticotropin injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63004-8710 Route of Administration INTRAMUSCULAR, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CORTICOTROPIN (UNII: K0U68Q2TXA) (CORTICOTROPIN - UNII:K0U68Q2TXA) CORTICOTROPIN 80 [USP'U] in 1 mL Inactive Ingredients Ingredient Name Strength GELATIN, UNSPECIFIED (UNII: 2G86QN327L) PHENOL (UNII: 339NCG44TV) CYSTEINE (UNII: K848JZ4886) SODIUM HYDROXIDE (UNII: 55X04QC32I) ACETIC ACID (UNII: Q40Q9N063P) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63004-8710-1 1 in 1 CARTON 01/07/2013 1 5 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product 2 NDC: 63004-8710-2 1 in 1 CARTON 01/07/2013 2 5 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product 3 NDC: 63004-8710-3 1 in 1 CARTON 01/07/2013 3 5 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022432 01/07/2013 Labeler - Mallinckrodt ARD LLC (625130828) Establishment Name Address ID/FEI Business Operations Cangene BioPharma, LLC 050783398 MANUFACTURE(63004-8710)
Trademark Results [Acthar]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ACTHAR 78649502 3093264 Live/Registered |
MALLINCKRODT ARD IP LIMITED 2005-06-13 |
 ACTHAR 71606253 0563816 Dead/Expired |
ARMOUR AND COMPANY 1950-11-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.