LINXMED-SP- lincomycin hydrochloride powder
LinxMed-SP by
Drug Labeling and Warnings
LinxMed-SP by is a Animal medication manufactured, distributed, or labeled by Bimeda, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
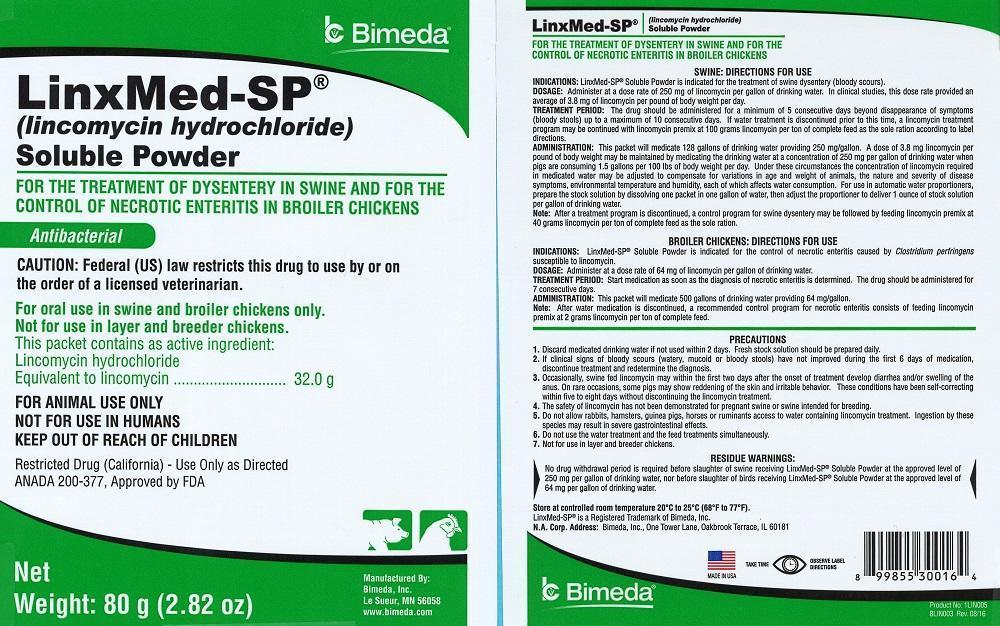
LinxMed-SP®
(lincomycin hydrochloride) Soluble Powder
FOR THE TREATMENT OF DYSENTERY IN SWINE AND FOR THE CONTROL OF NECROTIC ENTERITIS IN BROILER CHICKENS
Antibacterial
CAUTION: Federal (US) law restricts this drug to use by or on the order of a licensed veterinarian.
For oral use in swine and broiler chickens only.
Not for use in layer and breeder chickens.
This packet contains as active ingredient:
Lincomycin hydrochloride Equivalent to lincomycin.................32.0 g
FOR ANIMAL USE ONLY
NOT FOR USE IN HUMANS
KEEP OUT OF REACH OF CHILDREN
Restricted Drug - Use Only as Directed (California)
ANADA 200-377, Approved by FDA
Net Weight: 80 gm (2.82 oz)
-
INDICATIONS & USAGE
SWINE: DIRECTIONS FOR USE
INDICATIONS: LinxMed-SP® Soluble Powder is indicated for the treatment of swine dysentery (bloody scours).
DOSAGE: Administer at a dose rate of 250 mg of lincomycin per gallon of drinking water. In clinical studies, this dose rate provided an average of 3.8 mg of lincomycin per pound of body weight per day.
TREATMENT PERIOD: The drug should be administered for a minimum of 5 consecutive days beyond disappearance of symptoms (bloody stools) up to a maximum of 10 consecutive days. If water treatment is discontinued prior to this time, a lincomycin treatment program may be continued with lincomycin premix at 100 grams lincomycin per ton of complete feed as the sole ration according to label directions.
ADMINISTRATION: This packet will medicate 128 gallons of drinking water providing 250 mg/gallon. A dose of 3.8 mg lincomycin per pound of body weight may be maintained by medicating the drinking water at a concentration of 250 mg per gallon of drinking water when pigs are consuming 1.5 gallons per 100 lbs of body weight per day. Under these circumstances the concentration of lincomycin required in medicated water may be adjusted to compensate for variations in age and weight of animals, the nature and severity of disease symptoms, environmental temperature and humidity, each of which affects water consumption. For use in automatic water proportioners, prepare the stock solution by dissolving one packet in one gallon of water, then adjust the proportioner to deliver 1 ounce of stock solution per gallon of drinking water.
Note: After a treatment program is discontinued, a control program for swine dysentery may be followed by feeding lincomycin premix at 40 grams lincomycin per ton of complete feed as the sole ration.
BROILER CHICKENS:DIRECTIONS FOR USE
INDICATIONS: LinxMed-SP® Soluble Powder is indicated for the control of necrotic enteritis caused by Clostridium perfringens susceptible to lincomycin.
DOSAGE: Administer at a dose rate of 64 mg of lincomycin per gallon of drinking water.
TREATMENT PERIOD: Start medication as soon as the diagnosis of necrotic enteritis is determined. The drug should be administered for 7 consecutive days.
ADMINISTRATION: This packet will medicate 500 gallons of drinking water providing 64 mg/gallon.
Note: After water medication is discontinued, a recommended control program for necrotic enteritis consists of feeding lincomycin premix at 2 grams lincomycin per ton of complete feed.
-
PRECAUTIONS
PRECAUTIONS
1. Discard medicated drinking water if not used within 2 days. Fresh stock solution should be prepared daily.
2. If clinical signs of bloody scours (watery, mucoid or bloody stools) have not improved during the first 6 days of medication, discontinue treatment and redetermine the diagnosis.
3. Occasionally, swine fed lincomycin may within the first two days after the onset of treatment develop diarrhea and/or swelling of the anus. On rare occasions, some pigs may show reddening of the skin and irritable behavior. These conditions have been self-correcting within five to eight days without discontinuing the lincomycin treatment.
4. The safety of lincomycin has not been demonstrated for pregnant swine or swine intended for breeding.
5. Do not allow rabbits, hamsters, guinea pigs, horses or ruminants access to water containing lincomycin treatment. Ingestion by these species may result in severe gastrointestinal effects.
6. Do not use the water treatment and the feed treatments simultaneously.
7. Not for use in layer and breeder chickens.
-
RESIDUE WARNING
RESIDUE WARNINGS:
No drug withdrawal period is required before slaughter of swine receiving LinxMed-SP® Soluble Powder at the approved level of 250 mg per gallon of drinking water, nor before slaughter of birds receiving LinxMed-SP® Soluble Powder at the approved level of 64 mg per gallon of drinking water.
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LINXMED-SP
lincomycin hydrochloride powderProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 61133-5482 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Lincomycin Hydrochloride (UNII: M6T05Z2B68) (Lincomycin - UNII:BOD072YW0F) Lincomycin 32 g in 80 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61133-5482-2 80 g in 1 POUCH 2 NDC: 61133-5482-1 40 g in 1 POUCH 3 NDC: 61133-5482-3 907.18 g in 1 POUCH 4 NDC: 61133-5482-4 480 g in 1 POUCH 5 NDC: 61133-5482-5 160 g in 1 POUCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200377 12/06/2004 Labeler - Bimeda, Inc. (060492923) Registrant - Bimeda, Inc. (060492923) Establishment Name Address ID/FEI Business Operations Bimeda, Inc. 060492923 manufacture
Trademark Results [LinxMed-SP]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LINXMED-SP 77193175 3541054 Live/Registered |
BIMEDA, INC. 2007-05-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.