PROSCAR- finasteride tablet, film coated
PROSCAR by
Drug Labeling and Warnings
PROSCAR by is a Prescription medication manufactured, distributed, or labeled by Merck Sharp & Dohme Corp.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PROSCAR safely and effectively. See full prescribing information for PROSCAR.
PROSCAR® (finasteride) Tablets
Initial U.S. Approval: 1992INDICATIONS AND USAGE
PROSCAR, is a 5α-reductase inhibitor, indicated for the treatment of symptomatic benign prostatic hyperplasia (BPH) in men with an enlarged prostate to (1.1):
- Improve symptoms
- Reduce the risk of acute urinary retention
- Reduce the risk of the need for surgery including transurethral resection of the prostate (TURP) and prostatectomy.
PROSCAR administered in combination with the alpha-blocker doxazosin is indicated to reduce the risk of symptomatic progression of BPH (a confirmed ≥4 point increase in American Urological Association (AUA) symptom score) (1.2).
Limitations of Use: PROSCAR is not approved for the prevention of prostate cancer (1.3).DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
5-mg film-coated tablets (3).
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- PROSCAR reduces serum prostate specific antigen (PSA) levels by approximately 50%. However, any confirmed increase in PSA while on PROSCAR may signal the presence of prostate cancer and should be evaluated, even if those values are still within the normal range for men not taking a 5α-reductase inhibitor (5.1).
- PROSCAR may increase the risk of high-grade prostate cancer (5.2, 6.1).
- Women should not handle crushed or broken PROSCAR tablets when they are pregnant or may potentially be pregnant due to potential risk to a male fetus (5.3, 8.1, 16).
- PROSCAR is not indicated for use in pediatric patients or women (5.4, 8.1, 8.3, 8.4, 12.3).
- Prior to initiating treatment with PROSCAR for BPH, consideration should be given to other urological conditions that may cause similar symptoms (5.6).
ADVERSE REACTIONS
The drug-related adverse reactions, reported in ≥1% in patients treated with PROSCAR and greater than in patients treated with placebo over a 4-year study are: impotence, decreased libido, decreased volume of ejaculate, breast enlargement, breast tenderness and rash (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2013
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Monotherapy
1.2 Combination with Alpha-Blocker
1.3 Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Monotherapy
2.2 Combination with Alpha-Blocker
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Effects on Prostate Specific Antigen (PSA) and the Use of PSA in Prostate Cancer Detection
5.2 Increased Risk of High-Grade Prostate Cancer
5.3 Exposure of Women — Risk to Male Fetus
5.4 Pediatric Patients and Women
5.5 Effect on Semen Characteristics
5.6 Consideration of Other Urological Conditions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Cytochrome P450-Linked Drug Metabolizing Enzyme System
7.2 Other Concomitant Therapy
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Monotherapy
14.2 Combination with Alpha-Blocker Therapy
14.3 Summary of Clinical Studies
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Increased Risk of High-Grade Prostate Cancer
17.2 Exposure of Women — Risk to Male Fetus
17.3 Additional Instructions
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Monotherapy
PROSCAR® is indicated for the treatment of symptomatic benign prostatic hyperplasia (BPH) in men with an enlarged prostate to:
- Improve symptoms
- Reduce the risk of acute urinary retention
- Reduce the risk of the need for surgery including transurethral resection of the prostate (TURP) and prostatectomy.
-
2 DOSAGE AND ADMINISTRATION
PROSCAR may be administered with or without meals.
2.1 Monotherapy
The recommended dose of PROSCAR is one tablet (5 mg) taken once a day [see Clinical Studies (14.1)].
2.2 Combination with Alpha-Blocker
The recommended dose of PROSCAR is one tablet (5 mg) taken once a day in combination with the alpha-blocker doxazosin [see Clinical Studies (14.2)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
PROSCAR is contraindicated in the following:
- Hypersensitivity to any component of this medication.
- Pregnancy. Finasteride use is contraindicated in women when they are or may potentially be pregnant. Because of the ability of Type II 5α-reductase inhibitors to inhibit the conversion of testosterone to 5α-dihydrotestosterone (DHT), finasteride may cause abnormalities of the external genitalia of a male fetus of a pregnant woman who receives finasteride. If this drug is used during pregnancy, or if pregnancy occurs while taking this drug, the pregnant woman should be apprised of the potential hazard to the male fetus. [See also Warnings and Precautions (5.3), Use in Specific Populations (8.1), How Supplied/Storage and Handling (16) and Patient Counseling Information (17.2).] In female rats, low doses of finasteride administered during pregnancy have produced abnormalities of the external genitalia in male offspring.
-
5 WARNINGS AND PRECAUTIONS
5.1 Effects on Prostate Specific Antigen (PSA) and the Use of PSA in Prostate Cancer Detection
In clinical studies, PROSCAR reduced serum PSA concentration by approximately 50% within six months of treatment. This decrease is predictable over the entire range of PSA values in patients with symptomatic BPH, although it may vary in individuals.
For interpretation of serial PSAs in men taking PROSCAR, a new PSA baseline should be established at least six months after starting treatment and PSA monitored periodically thereafter. Any confirmed increase from the lowest PSA value while on PROSCAR may signal the presence of prostate cancer and should be evaluated, even if PSA levels are still within the normal range for men not taking a 5α-reductase inhibitor. Non-compliance with PROSCAR therapy may also affect PSA test results. To interpret an isolated PSA value in patients treated with PROSCAR for six months or more, PSA values should be doubled for comparison with normal ranges in untreated men. These adjustments preserve the utility of PSA to detect prostate cancer in men treated with PROSCAR.
PROSCAR may also cause decreases in serum PSA in the presence of prostate cancer.
The ratio of free to total PSA (percent free PSA) remains constant even under the influence of PROSCAR. If clinicians elect to use percent free PSA as an aid in the detection of prostate cancer in men undergoing finasteride therapy, no adjustment to its value appears necessary.
5.2 Increased Risk of High-Grade Prostate Cancer
Men aged 55 and over with a normal digital rectal examination and PSA ≤3.0 ng/mL at baseline taking finasteride 5 mg/day in the 7-year Prostate Cancer Prevention Trial (PCPT) had an increased risk of Gleason score 8-10 prostate cancer (finasteride 1.8% vs placebo 1.1%). [See Indications and Usage (1.3) and Adverse Reactions (6.1).] Similar results were observed in a 4-year placebo-controlled clinical trial with another 5α-reductase inhibitor (dutasteride, AVODART) (1% dutasteride vs 0.5% placebo). 5α-reductase inhibitors may increase the risk of development of high-grade prostate cancer. Whether the effect of 5α-reductase inhibitors to reduce prostate volume, or study-related factors, impacted the results of these studies has not been established.
5.3 Exposure of Women — Risk to Male Fetus
Women should not handle crushed or broken PROSCAR tablets when they are pregnant or may potentially be pregnant because of the possibility of absorption of finasteride and the subsequent potential risk to a male fetus. PROSCAR tablets are coated and will prevent contact with the active ingredient during normal handling, provided that the tablets have not been broken or crushed. [See Contraindications (4), Use in Specific Populations (8.1), Clinical Pharmacology (12.3), How Supplied/Storage and Handling (16) and Patient Counseling Information (17.2).]
5.4 Pediatric Patients and Women
PROSCAR is not indicated for use in pediatric patients [see Use in Specific Populations (8.4) and Clinical Pharmacology (12.3)] or women [see also Warnings and Precautions (5.3), Use in Specific Populations (8.1), Clinical Pharmacology (12.3), How Supplied/Storage and Handling (16) and Patient Counseling Information (17.2)].
5.5 Effect on Semen Characteristics
Treatment with PROSCAR for 24 weeks to evaluate semen parameters in healthy male volunteers revealed no clinically meaningful effects on sperm concentration, mobility, morphology, or pH. A 0.6 mL (22.1%) median decrease in ejaculate volume with a concomitant reduction in total sperm per ejaculate was observed. These parameters remained within the normal range and were reversible upon discontinuation of therapy with an average time to return to baseline of 84 weeks.
5.6 Consideration of Other Urological Conditions
Prior to initiating treatment with PROSCAR, consideration should be given to other urological conditions that may cause similar symptoms. In addition, prostate cancer and BPH may coexist.
Patients with large residual urinary volume and/or severely diminished urinary flow should be carefully monitored for obstructive uropathy. These patients may not be candidates for finasteride therapy.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
PROSCAR is generally well tolerated; adverse reactions usually have been mild and transient.
4-Year Placebo-Controlled Study (PLESS)
In PLESS, 1524 patients treated with PROSCAR and 1516 patients treated with placebo were evaluated for safety over a period of 4 years. The most frequently reported adverse reactions were related to sexual function. 3.7% (57 patients) treated with PROSCAR and 2.1% (32 patients) treated with placebo discontinued therapy as a result of adverse reactions related to sexual function, which are the most frequently reported adverse reactions.
Table 1 presents the only clinical adverse reactions considered possibly, probably or definitely drug related by the investigator, for which the incidence on PROSCAR was ≥1% and greater than placebo over the 4 years of the study. In years 2-4 of the study, there was no significant difference between treatment groups in the incidences of impotence, decreased libido and ejaculation disorder.
Table 1: Drug-Related Adverse Experiences Year 1
(%)Years 2, 3 and 4*
(%)Finasteride Placebo Finasteride Placebo N = 1524 and 1516, finasteride vs placebo, respectively - * Combined Years 2-4
Impotence 8.1 3.7 5.1 5.1 Decreased
Libido6.4 3.4 2.6 2.6 Decreased
Volume of
Ejaculate
3.7
0.8
1.5
0.5Ejaculation
Disorder0.8 0.1 0.2 0.1 Breast
Enlargement0.5 0.1 1.8 1.1 Breast
Tenderness0.4 0.1 0.7 0.3 Rash 0.5 0.2 0.5 0.1 Phase III Studies and 5-Year Open Extensions
The adverse experience profile in the 1-year, placebo-controlled, Phase III studies, the 5-year open extensions, and PLESS were similar.
Medical Therapy of Prostatic Symptoms (MTOPS) Study
In the MTOPS study, 3047 men with symptomatic BPH were randomized to receive PROSCAR 5 mg/day (n=768), doxazosin 4 or 8 mg/day (n=756), the combination of PROSCAR 5 mg/day and doxazosin 4 or 8 mg/day (n=786), or placebo (n=737) for 4 to 6 years. [See Clinical Studies (14.2).]
The incidence rates of drug-related adverse experiences reported by ≥2% of patients in any treatment group in the MTOPS Study are listed in Table 2.
The individual adverse effects which occurred more frequently in the combination group compared to either drug alone were: asthenia, postural hypotension, peripheral edema, dizziness, decreased libido, rhinitis, abnormal ejaculation, impotence and abnormal sexual function (see Table 2). Of these, the incidence of abnormal ejaculation in patients receiving combination therapy was comparable to the sum of the incidences of this adverse experience reported for the two monotherapies.
Combination therapy with finasteride and doxazosin was associated with no new clinical adverse experience.
Four patients in MTOPS reported the adverse experience breast cancer. Three of these patients were on finasteride only and one was on combination therapy. [See Long-Term Data.]
The MTOPS Study was not specifically designed to make statistical comparisons between groups for reported adverse experiences. In addition, direct comparisons of safety data between the MTOPS study and previous studies of the single agents may not be appropriate based upon differences in patient population, dosage or dose regimen, and other procedural and study design elements.
Table 2: Incidence ≥2% in One or More Treatment Groups Drug-Related Clinical Adverse Experiences in MTOPS Adverse Experience Placebo
(N=737)
(%)Doxazosin
4 mg or 8 mg*
(N=756)
(%)Finasteride
(N=768)
(%)Combination
(N=786)
(%)- * Doxazosin dose was achieved by weekly titration (1 to 2 to 4 to 8 mg). The final tolerated dose (4 mg or 8 mg) was administered at end-Week 4. Only those patients tolerating at least 4 mg were kept on doxazosin. The majority of patients received the 8-mg dose over the duration of the study.
Body as a whole Asthenia 7.1 15.7 5.3 16.8 Headache 2.3 4.1 2.0 2.3 Cardiovascular Hypotension 0.7 3.4 1.2 1.5 Postural Hypotension 8.0 16.7 9.1 17.8 Metabolic and Nutritional Peripheral Edema 0.9 2.6 1.3 3.3 Nervous Dizziness 8.1 17.7 7.4 23.2 Libido Decreased 5.7 7.0 10.0 11.6 Somnolence 1.5 3.7 1.7 3.1 Respiratory Dyspnea 0.7 2.1 0.7 1.9 Rhinitis 0.5 1.3 1.0 2.4 Urogenital Abnormal Ejaculation 2.3 4.5 7.2 14.1 Gynecomastia 0.7 1.1 2.2 1.5 Impotence 12.2 14.4 18.5 22.6 Sexual Function Abnormal 0.9 2.0 2.5 3.1 High-Grade Prostate Cancer
The PCPT trial was a 7-year randomized, double-blind, placebo-controlled trial that enrolled 18,882 men ≥55 years of age with a normal digital rectal examination and a PSA ≤3.0 ng/mL. Men received either PROSCAR (finasteride 5 mg) or placebo daily. Patients were evaluated annually with PSA and digital rectal exams. Biopsies were performed for elevated PSA, an abnormal digital rectal exam, or the end of study. The incidence of Gleason score 8-10 prostate cancer was higher in men treated with finasteride (1.8%) than in those treated with placebo (1.1%) [see Indications and Usage (1.3) and Warnings and Precautions (5.2)]. In a 4-year placebo-controlled clinical trial with another 5α-reductase inhibitor (dutasteride, AVODART), similar results for Gleason score 8-10 prostate cancer were observed (1% dutasteride vs 0.5% placebo).
No clinical benefit has been demonstrated in patients with prostate cancer treated with PROSCAR.
Breast Cancer
During the 4- to 6-year placebo- and comparator-controlled MTOPS study that enrolled 3047 men, there were 4 cases of breast cancer in men treated with finasteride but no cases in men not treated with finasteride. During the 4-year, placebo-controlled PLESS study that enrolled 3040 men, there were 2 cases of breast cancer in placebo-treated men but no cases in men treated with finasteride. During the 7-year placebo-controlled Prostate Cancer Prevention Trial (PCPT) that enrolled 18,882 men, there was 1 case of breast cancer in men treated with finasteride, and 1 case of breast cancer in men treated with placebo. The relationship between long-term use of finasteride and male breast neoplasia is currently unknown.
Sexual Function
There is no evidence of increased sexual adverse experiences with increased duration of treatment with PROSCAR. New reports of drug-related sexual adverse experiences decreased with duration of therapy.
6.2 Postmarketing Experience
The following additional adverse events have been reported in postmarketing experience with PROSCAR. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
- - hypersensitivity reactions, such as pruritus, urticaria, and angioedema (including swelling of the lips, tongue, throat, and face)
- - testicular pain
- - sexual dysfunction that continued after discontinuation of treatment, including erectile dysfunction, decreased libido and ejaculation disorders (e.g. reduced ejaculate volume). These events were reported rarely in men taking PROSCAR for the treatment of BPH. Most men were older and were taking concomitant medications and/or had co-morbid conditions. The independent role of PROSCAR in these events is unknown.
- - male infertility and/or poor seminal quality were reported rarely in men taking PROSCAR for the treatment of BPH. Normalization or improvement of poor seminal quality has been reported after discontinuation of finasteride. The independent role of PROSCAR in these events is unknown.
- - depression
- - male breast cancer.
The following additional adverse event related to sexual dysfunction that continued after discontinuation of treatment has been reported in postmarketing experience with finasteride at lower doses used to treat male pattern baldness. Because the event is reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate its frequency or establish a causal relationship to drug exposure:
- - orgasm disorders
-
7 DRUG INTERACTIONS
7.1 Cytochrome P450-Linked Drug Metabolizing Enzyme System
No drug interactions of clinical importance have been identified. Finasteride does not appear to affect the cytochrome P450-linked drug metabolizing enzyme system. Compounds that have been tested in man have included antipyrine, digoxin, propranolol, theophylline, and warfarin and no clinically meaningful interactions were found.
7.2 Other Concomitant Therapy
Although specific interaction studies were not performed, PROSCAR was concomitantly used in clinical studies with acetaminophen, acetylsalicylic acid, α-blockers, angiotensin-converting enzyme (ACE) inhibitors, analgesics, anti-convulsants, beta-adrenergic blocking agents, diuretics, calcium channel blockers, cardiac nitrates, HMG-CoA reductase inhibitors, nonsteroidal anti-inflammatory drugs (NSAIDs), benzodiazepines, H2 antagonists and quinolone anti-infectives without evidence of clinically significant adverse interactions.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category X. [See Contraindications (4).]
PROSCAR is contraindicated for use in women who are or may become pregnant. PROSCAR is a Type II 5α-reductase inhibitor that prevents conversion of testosterone to 5α-dihydrotestosterone (DHT), a hormone necessary for normal development of male genitalia. In animal studies, finasteride caused abnormal development of external genitalia in male fetuses. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the male fetus.
Abnormal male genital development is an expected consequence when conversion of testosterone to 5α-dihydrotestosterone (DHT) is inhibited by 5α-reductase inhibitors. These outcomes are similar to those reported in male infants with genetic 5α-reductase deficiency. Women could be exposed to finasteride through contact with crushed or broken PROSCAR tablets or semen from a male partner taking PROSCAR. With regard to finasteride exposure through the skin, PROSCAR tablets are coated and will prevent skin contact with finasteride during normal handling if the tablets have not been crushed or broken. Women who are pregnant or may become pregnant should not handle crushed or broken PROSCAR tablets because of possible exposure of a male fetus. If a pregnant woman comes in contact with crushed or broken PROSCAR tablets, the contact area should be washed immediately with soap and water. With regard to potential finasteride exposure through semen, two studies have been conducted in men receiving PROSCAR 5 mg/day that measured finasteride concentrations in semen [see Clinical Pharmacology (12.3)].
In an embryo-fetal development study, pregnant rats received finasteride during the period of major organogenesis (gestation days 6 to 17). At maternal doses of oral finasteride approximately 0.1 to 86 times the maximum recommended human dose (MRHD) of 5 mg/day (based on AUC at animal doses of 0.1 to 100 mg/kg/day) there was a dose-dependent increase in hypospadias that occurred in 3.6 to 100% of male offspring. Exposure multiples were estimated using data from nonpregnant rats. Days 16 to 17 of gestation is a critical period in male fetal rats for differentiation of the external genitalia. At oral maternal doses approximately 0.03 times the MRHD (based on AUC at animal dose of 0.03 mg/kg/day), male offspring had decreased prostatic and seminal vesicular weights, delayed preputial separation and transient nipple development. Decreased anogenital distance occurred in male offspring of pregnant rats that received approximately 0.003 times the MRHD (based on AUC at animal dose of 0.003 mg/kg/day). No abnormalities were observed in female offspring at any maternal dose of finasteride.
No developmental abnormalities were observed in the offspring of untreated females mated with finasteride treated male rats that received approximately 61 times the MRHD (based on AUC at animal dose of 80 mg/kg/day). Slightly decreased fertility was observed in male offspring after administration of about 3 times the MRHD (based on AUC at animal dose of 3 mg/kg/day) to female rats during late gestation and lactation. No effects on fertility were seen in female offspring under these conditions.
No evidence of male external genital malformations or other abnormalities were observed in rabbit fetuses exposed to finasteride during the period of major organogenesis (gestation days 6-18) at maternal oral doses up to 100 mg/kg/day, (finasteride exposure levels were not measured in rabbits). However, this study may not have included the critical period for finasteride effects on development of male external genitalia in the rabbit.
The fetal effects of maternal finasteride exposure during the period of embryonic and fetal development were evaluated in the rhesus monkey (gestation days 20-100), in a species and development period more predictive of specific effects in humans than the studies in rats and rabbits. Intravenous administration of finasteride to pregnant monkeys at doses as high as 800 ng/day (estimated maximal blood concentration of 1.86 ng/mL or about 143 times the highest estimated exposure of pregnant women to finasteride from semen of men taking 5 mg/day) resulted in no abnormalities in male fetuses. In confirmation of the relevance of the rhesus model for human fetal development, oral administration of a dose of finasteride (2 mg/kg/day or approximately 18,000 times the highest estimated blood levels of finasteride from semen of men taking 5 mg/day) to pregnant monkeys resulted in external genital abnormalities in male fetuses. No other abnormalities were observed in male fetuses and no finasteride-related abnormalities were observed in female fetuses at any dose.
8.3 Nursing Mothers
PROSCAR is not indicated for use in women.
It is not known whether finasteride is excreted in human milk.
8.4 Pediatric Use
PROSCAR is not indicated for use in pediatric patients.
Safety and effectiveness in pediatric patients have not been established.
8.5 Geriatric Use
Of the total number of subjects included in PLESS, 1480 and 105 subjects were 65 and over and 75 and over, respectively. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients. No dosage adjustment is necessary in the elderly [see Clinical Pharmacology (12.3) and Clinical Studies (14)].
8.6 Hepatic Impairment
Caution should be exercised in the administration of PROSCAR in those patients with liver function abnormalities, as finasteride is metabolized extensively in the liver [see Clinical Pharmacology (12.3)].
8.7 Renal Impairment
No dosage adjustment is necessary in patients with renal impairment [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Patients have received single doses of PROSCAR up to 400 mg and multiple doses of PROSCAR up to 80 mg/day for three months without adverse effects. Until further experience is obtained, no specific treatment for an overdose with PROSCAR can be recommended.
Significant lethality was observed in male and female mice at single oral doses of 1500 mg/m2 (500 mg/kg) and in female and male rats at single oral doses of 2360 mg/m2 (400 mg/kg) and 5900 mg/m2 (1000 mg/kg), respectively.
-
11 DESCRIPTION
PROSCAR (finasteride), a synthetic 4-azasteroid compound, is a specific inhibitor of steroid Type II 5α-reductase, an intracellular enzyme that converts the androgen testosterone into 5α-dihydrotestosterone (DHT).
Finasteride is 4-azaandrost-1-ene-17-carboxamide, N-(1,1-dimethylethyl)-3-oxo-,(5α,17ß)-. The empirical formula of finasteride is C23H36N2O2 and its molecular weight is 372.55. Its structural formula is:
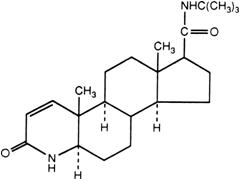
Finasteride is a white crystalline powder with a melting point near 250°C. It is freely soluble in chloroform and in lower alcohol solvents, but is practically insoluble in water.
PROSCAR (finasteride) tablets for oral administration are film-coated tablets that contain 5 mg of finasteride and the following inactive ingredients: hydrous lactose, microcrystalline cellulose, pregelatinized starch, sodium starch glycolate, hydroxypropyl cellulose LF, hydroxypropyl methylcellulose, titanium dioxide, magnesium stearate, talc, docusate sodium, FD&C Blue 2 aluminum lake and yellow iron oxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The development and enlargement of the prostate gland is dependent on the potent androgen, 5α-dihydrotestosterone (DHT). Type II 5α-reductase metabolizes testosterone to DHT in the prostate gland, liver and skin. DHT induces androgenic effects by binding to androgen receptors in the cell nuclei of these organs.
Finasteride is a competitive and specific inhibitor of Type II 5α-reductase with which it slowly forms a stable enzyme complex. Turnover from this complex is extremely slow (t½ ~ 30 days). This has been demonstrated both in vivo and in vitro. Finasteride has no affinity for the androgen receptor. In man, the 5α-reduced steroid metabolites in blood and urine are decreased after administration of finasteride.
12.2 Pharmacodynamics
In man, a single 5-mg oral dose of PROSCAR produces a rapid reduction in serum DHT concentration, with the maximum effect observed 8 hours after the first dose. The suppression of DHT is maintained throughout the 24-hour dosing interval and with continued treatment. Daily dosing of PROSCAR at 5 mg/day for up to 4 years has been shown to reduce the serum DHT concentration by approximately 70%. The median circulating level of testosterone increased by approximately 10-20% but remained within the physiologic range. In a separate study in healthy men treated with finasteride 1 mg per day (n=82) or placebo (n=69), mean circulating levels of testosterone and estradiol were increased by approximately 15% as compared to baseline, but these remained within the physiologic range.
In patients receiving PROSCAR 5 mg/day, increases of about 10% were observed in luteinizing hormone (LH) and follicle-stimulating hormone (FSH), but levels remained within the normal range. In healthy volunteers, treatment with PROSCAR did not alter the response of LH and FSH to gonadotropin-releasing hormone indicating that the hypothalamic-pituitary-testicular axis was not affected.
In patients with BPH, PROSCAR has no effect on circulating levels of cortisol, prolactin, thyroid-stimulating hormone, or thyroxine. No clinically meaningful effect was observed on the plasma lipid profile (i.e., total cholesterol, low density lipoproteins, high density lipoproteins and triglycerides) or bone mineral density.
Adult males with genetically inherited Type II 5α-reductase deficiency also have decreased levels of DHT. Except for the associated urogenital defects present at birth, no other clinical abnormalities related to Type II 5α-reductase deficiency have been observed in these individuals. These individuals have a small prostate gland throughout life and do not develop BPH.
In patients with BPH treated with finasteride (1-100 mg/day) for 7-10 days prior to prostatectomy, an approximate 80% lower DHT content was measured in prostatic tissue removed at surgery, compared to placebo; testosterone tissue concentration was increased up to 10 times over pretreatment levels, relative to placebo. Intraprostatic content of PSA was also decreased.
In healthy male volunteers treated with PROSCAR for 14 days, discontinuation of therapy resulted in a return of DHT levels to pretreatment levels in approximately 2 weeks. In patients treated for three months, prostate volume, which declined by approximately 20%, returned to close to baseline value after approximately three months of discontinuation of therapy.
12.3 Pharmacokinetics
Absorption
In a study of 15 healthy young subjects, the mean bioavailability of finasteride 5-mg tablets was 63% (range 34-108%), based on the ratio of area under the curve (AUC) relative to an intravenous (IV) reference dose. Maximum finasteride plasma concentration averaged 37 ng/mL (range, 27-49 ng/mL) and was reached 1-2 hours postdose. Bioavailability of finasteride was not affected by food.
Distribution
Mean steady-state volume of distribution was 76 liters (range, 44-96 liters). Approximately 90% of circulating finasteride is bound to plasma proteins. There is a slow accumulation phase for finasteride after multiple dosing. After dosing with 5 mg/day of finasteride for 17 days, plasma concentrations of finasteride were 47 and 54% higher than after the first dose in men 45-60 years old (n=12) and ≥70 years old (n=12), respectively. Mean trough concentrations after 17 days of dosing were 6.2 ng/mL (range, 2.4-9.8 ng/mL) and 8.1 ng/mL (range, 1.8-19.7 ng/mL), respectively, in the two age groups. Although steady state was not reached in this study, mean trough plasma concentration in another study in patients with BPH (mean age, 65 years) receiving 5 mg/day was 9.4 ng/mL (range, 7.1-13.3 ng/mL; n=22) after over a year of dosing.
Finasteride has been shown to cross the blood brain barrier but does not appear to distribute preferentially to the CSF.
In 2 studies of healthy subjects (n=69) receiving PROSCAR 5 mg/day for 6-24 weeks, finasteride concentrations in semen ranged from undetectable (<0.1 ng/mL) to 10.54 ng/mL. In an earlier study using a less sensitive assay, finasteride concentrations in the semen of 16 subjects receiving PROSCAR 5 mg/day ranged from undetectable (<1.0 ng/mL) to 21 ng/mL. Thus, based on a 5-mL ejaculate volume, the amount of finasteride in semen was estimated to be 50- to 100-fold less than the dose of finasteride (5 μg) that had no effect on circulating DHT levels in men [see also Use in Specific Populations (8.1)].
Metabolism
Finasteride is extensively metabolized in the liver, primarily via the cytochrome P450 3A4 enzyme subfamily. Two metabolites, the t-butyl side chain monohydroxylated and monocarboxylic acid metabolites, have been identified that possess no more than 20% of the 5α-reductase inhibitory activity of finasteride.
Excretion
In healthy young subjects (n=15), mean plasma clearance of finasteride was 165 mL/min (range, 70-279 mL/min) and mean elimination half-life in plasma was 6 hours (range, 3-16 hours). Following an oral dose of 14C-finasteride in man (n=6), a mean of 39% (range, 32-46%) of the dose was excreted in the urine in the form of metabolites; 57% (range, 51-64%) was excreted in the feces.
The mean terminal half-life of finasteride in subjects ≥70 years of age was approximately 8 hours (range, 6-15 hours; n=12), compared with 6 hours (range, 4-12 hours; n=12) in subjects 45-60 years of age. As a result, mean AUC(0-24 hr) after 17 days of dosing was 15% higher in subjects ≥70 years of age than in subjects 45-60 years of age (p=0.02).
Table 3: Mean (SD) Pharmacokinetic Parameters in Healthy Young Subjects (n=15)
Mean (± SD) - * Range
Bioavailability 63% (34-108%)* Clearance (mL/min) 165 (55) Volume of Distribution (L) 76 (14) Half-Life (hours) 6.2 (2.1) Pediatric
Finasteride pharmacokinetics have not been investigated in patients <18 years of age.
Finasteride is not indicated for use in pediatric patients [see Warnings and Precautions (5.4), Use in Specific Populations (8.4)].
Gender
Finasteride is not indicated for use in women [see Contraindications (4), Warnings and Precautions (5.3 and 5.4), Use in Specific Populations (8.1), How Supplied/Storage and Handling (16) and Patient Counseling Information (17.2)].
Geriatric
No dosage adjustment is necessary in the elderly. Although the elimination rate of finasteride is decreased in the elderly, these findings are of no clinical significance. [See Clinical Pharmacology (12.3) and Use in Specific Populations (8.5).]
Table 4: Mean (SD) Noncompartmental Pharmacokinetic Parameters After Multiple Doses of 5 mg/day in Older Men
Mean (± SD)
45-60 years old (n=12) ≥70 years old (n=12) - * First-dose values; all other parameters are last-dose values
AUC (nghr/mL) 389 (98) 463 (186) Peak Concentration (ng/mL) 46.2 (8.7) 48.4 (14.7) Time to Peak (hours) 1.8 (0.7) 1.8 (0.6) Half-Life (hours)* 6.0 (1.5) 8.2 (2.5) Race
The effect of race on finasteride pharmacokinetics has not been studied.
Hepatic Impairment
The effect of hepatic impairment on finasteride pharmacokinetics has not been studied. Caution should be exercised in the administration of PROSCAR in those patients with liver function abnormalities, as finasteride is metabolized extensively in the liver.
Renal Impairment
No dosage adjustment is necessary in patients with renal impairment. In patients with chronic renal impairment, with creatinine clearances ranging from 9.0 to 55 mL/min, AUC, maximum plasma concentration, half-life, and protein binding after a single dose of 14C-finasteride were similar to values obtained in healthy volunteers. Urinary excretion of metabolites was decreased in patients with renal impairment. This decrease was associated with an increase in fecal excretion of metabolites. Plasma concentrations of metabolites were significantly higher in patients with renal impairment (based on a 60% increase in total radioactivity AUC). However, finasteride has been well tolerated in BPH patients with normal renal function receiving up to 80 mg/day for 12 weeks, where exposure of these patients to metabolites would presumably be much greater.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No evidence of a tumorigenic effect was observed in a 24-month study in Sprague-Dawley rats receiving doses of finasteride up to 160 mg/kg/day in males and 320 mg/kg/day in females. These doses produced respective systemic exposure in rats of 111 and 274 times those observed in man receiving the recommended human dose of 5 mg/day. All exposure calculations were based on calculated AUC(0-24 hr) for animals and mean AUC(0-24 hr) for man (0.4 μghr/mL).
In a 19-month carcinogenicity study in CD-1 mice, a statistically significant (p≤0.05) increase in the incidence of testicular Leydig cell adenomas was observed at 228 times the human exposure (250 mg/kg/day). In mice at 23 times the human exposure, estimated (25 mg/kg/day) and in rats at 39 times the human exposure (40 mg/kg/day) an increase in the incidence of Leydig cell hyperplasia was observed. A positive correlation between the proliferative changes in the Leydig cells and an increase in serum LH levels (2- to 3-fold above control) has been demonstrated in both rodent species treated with high doses of finasteride. No drug-related Leydig cell changes were seen in either rats or dogs treated with finasteride for 1 year at 30 and 350 times (20 mg/kg/day and 45 mg/kg/day, respectively) or in mice treated for 19 months at 2.3 times the human exposure, estimated (2.5 mg/kg/day).
No evidence of mutagenicity was observed in an in vitro bacterial mutagenesis assay, a mammalian cell mutagenesis assay, or in an in vitro alkaline elution assay. In an in vitro chromosome aberration assay, using Chinese hamster ovary cells, there was a slight increase in chromosome aberrations. These concentrations correspond to 4000-5000 times the peak plasma levels in man given a total dose of 5 mg. In an in vivo chromosome aberration assay in mice, no treatment-related increase in chromosome aberration was observed with finasteride at the maximum tolerated dose of 250 mg/kg/day (228 times the human exposure) as determined in the carcinogenicity studies.
In sexually mature male rabbits treated with finasteride at 543 times the human exposure (80 mg/kg/day) for up to 12 weeks, no effect on fertility, sperm count, or ejaculate volume was seen. In sexually mature male rats treated with 61 times the human exposure (80 mg/kg/day), there were no significant effects on fertility after 6 or 12 weeks of treatment; however, when treatment was continued for up to 24 or 30 weeks, there was an apparent decrease in fertility, fecundity and an associated significant decrease in the weights of the seminal vesicles and prostate. All these effects were reversible within 6 weeks of discontinuation of treatment. No drug-related effect on testes or on mating performance has been seen in rats or rabbits. This decrease in fertility in finasteride-treated rats is secondary to its effect on accessory sex organs (prostate and seminal vesicles) resulting in failure to form a seminal plug. The seminal plug is essential for normal fertility in rats and is not relevant in man.
-
14 CLINICAL STUDIES
14.1 Monotherapy
PROSCAR 5 mg/day was initially evaluated in patients with symptoms of BPH and enlarged prostates by digital rectal examination in two 1-year, placebo-controlled, randomized, double-blind studies and their 5-year open extensions.
PROSCAR was further evaluated in the PROSCAR Long-Term Efficacy and Safety Study (PLESS), a double-blind, randomized, placebo-controlled, 4-year, multicenter study. 3040 patients between the ages of 45 and 78, with moderate to severe symptoms of BPH and an enlarged prostate upon digital rectal examination, were randomized into the study (1524 to finasteride, 1516 to placebo) and 3016 patients were evaluable for efficacy. 1883 patients completed the 4-year study (1000 in the finasteride group, 883 in the placebo group).
Effect on Symptom Score
Symptoms were quantified using a score similar to the American Urological Association Symptom Score, which evaluated both obstructive symptoms (impairment of size and force of stream, sensation of incomplete bladder emptying, delayed or interrupted urination) and irritative symptoms (nocturia, daytime frequency, need to strain or push the flow of urine) by rating on a 0 to 5 scale for six symptoms and a 0 to 4 scale for one symptom, for a total possible score of 34.
Patients in PLESS had moderate to severe symptoms at baseline (mean of approximately 15 points on a 0-34 point scale). Patients randomized to PROSCAR who remained on therapy for 4 years had a mean (± 1 SD) decrease in symptom score of 3.3 (± 5.8) points compared with 1.3 (± 5.6) points in the placebo group. (See Figure 1.) A statistically significant improvement in symptom score was evident at 1 year in patients treated with PROSCAR vs placebo (–2.3 vs –1.6), and this improvement continued through Year 4.
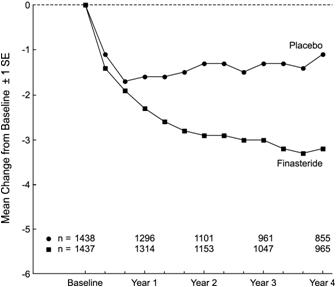
Figure 1: Symptom Score in PLESS
Results seen in earlier studies were comparable to those seen in PLESS. Although an early improvement in urinary symptoms was seen in some patients, a therapeutic trial of at least 6 months was generally necessary to assess whether a beneficial response in symptom relief had been achieved. The improvement in BPH symptoms was seen during the first year and maintained throughout an additional 5 years of open extension studies.
Effect on Acute Urinary Retention and the Need for Surgery
In PLESS, efficacy was also assessed by evaluating treatment failures. Treatment failure was prospectively defined as BPH-related urological events or clinical deterioration, lack of improvement and/or the need for alternative therapy. BPH-related urological events were defined as urological surgical intervention and acute urinary retention requiring catheterization. Complete event information was available for 92% of the patients. The following table (Table 5) summarizes the results.
Table 5: All Treatment Failures in PLESS
Patients (%)*
Event Placebo
N=1503Finasteride
N=1513Relative
Risk†95% CI P
Value†- * patients with multiple events may be counted more than once for each type of event
- † Hazard ratio based on log rank test
All Treatment Failures 37.1 26.2 0.68 (0.57 to 0.79) <0.001 Surgical
Interventions for
BPH10.1 4.6 0.45 (0.32 to 0.63) <0.001 Acute Urinary
Retention
Requiring
Catheterization6.6 2.8 0.43 (0.28 to 0.66) <0.001 Two consecutive
symptom scores
≥209.2 6.7 Bladder Stone 0.4 0.5 Incontinence 2.1 1.7 Renal Failure 0.5 0.6 UTI 5.7 4.9 Discontinuation
due to worsening
of BPH, lack of
improvement, or
to receive other
medical treatment21.8 13.3 Compared with placebo, PROSCAR was associated with a significantly lower risk for acute urinary retention or the need for BPH-related surgery [13.2% for placebo vs 6.6% for PROSCAR; 51% reduction in risk, 95% CI: (34 to 63%)]. Compared with placebo, PROSCAR was associated with a significantly lower risk for surgery [10.1% for placebo vs 4.6% for PROSCAR; 55% reduction in risk, 95% CI: (37 to 68%)] and with a significantly lower risk of acute urinary retention [6.6% for placebo vs 2.8% for PROSCAR; 57% reduction in risk, 95% CI: (34 to 72%)]; see Figures 2 and 3.
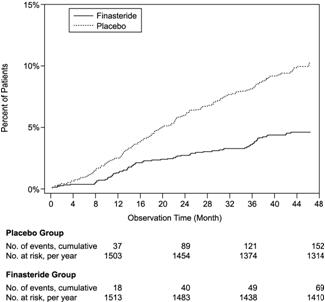
Figure 2: Percent of Patients Having Surgery for BPH, Including TURP
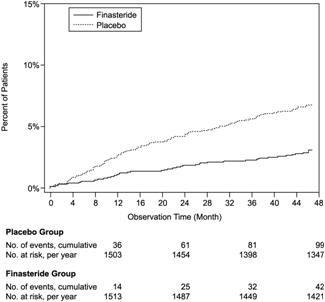
Figure 3: Percent of Patients Developing Acute Urinary Retention (Spontaneous and Precipitated)
Effect on Maximum Urinary Flow Rate
In the patients in PLESS who remained on therapy for the duration of the study and had evaluable urinary flow data, PROSCAR increased maximum urinary flow rate by 1.9 mL/sec compared with 0.2 mL/sec in the placebo group.
There was a clear difference between treatment groups in maximum urinary flow rate in favor of PROSCAR by month 4 (1.0 vs 0.3 mL/sec) which was maintained throughout the study. In the earlier 1-year studies, increase in maximum urinary flow rate was comparable to PLESS and was maintained through the first year and throughout an additional 5 years of open extension studies.
Effect on Prostate Volume
In PLESS, prostate volume was assessed yearly by magnetic resonance imaging (MRI) in a subset of patients. In patients treated with PROSCAR who remained on therapy, prostate volume was reduced compared with both baseline and placebo throughout the 4-year study. PROSCAR decreased prostate volume by 17.9% (from 55.9 cc at baseline to 45.8 cc at 4 years) compared with an increase of 14.1% (from 51.3 cc to 58.5 cc) in the placebo group (p<0.001). (See Figure 4.)
Results seen in earlier studies were comparable to those seen in PLESS. Mean prostate volume at baseline ranged between 40-50 cc. The reduction in prostate volume was seen during the first year and maintained throughout an additional five years of open extension studies.
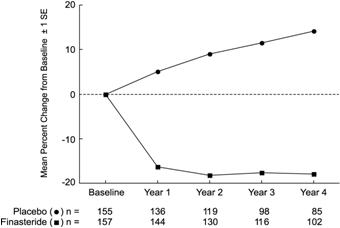
Figure 4: Prostate Volume in PLESS
Prostate Volume as a Predictor of Therapeutic Response
A meta-analysis combining 1-year data from seven double-blind, placebo-controlled studies of similar design, including 4491 patients with symptomatic BPH, demonstrated that, in patients treated with PROSCAR, the magnitude of symptom response and degree of improvement in maximum urinary flow rate were greater in patients with an enlarged prostate at baseline.
14.2 Combination with Alpha-Blocker Therapy
The Medical Therapy of Prostatic Symptoms (MTOPS) Trial was a double-blind, randomized, placebo-controlled, multicenter, 4- to 6-year study (average 5 years) in 3047 men with symptomatic BPH, who were randomized to receive PROSCAR 5 mg/day (n=768), doxazosin 4 or 8 mg/day (n=756), the combination of PROSCAR 5 mg/day and doxazosin 4 or 8 mg/day (n=786), or placebo (n=737). All participants underwent weekly titration of doxazosin (or its placebo) from 1 to 2 to 4 to 8 mg/day. Only those who tolerated the 4 or 8 mg dose level were kept on doxazosin (or its placebo) in the study. The participant's final tolerated dose (either 4 mg or 8 mg) was administered beginning at end-Week 4. The final doxazosin dose was administered once per day, at bedtime.
The mean patient age at randomization was 62.6 years (±7.3 years). Patients were Caucasian (82%), African American (9%), Hispanic (7%), Asian (1%) or Native American (<1%). The mean duration of BPH symptoms was 4.7 years (±4.6 years). Patients had moderate to severe BPH symptoms at baseline with a mean AUA symptom score of approximately 17 out of 35 points. Mean maximum urinary flow rate was 10.5 mL/sec (±2.6 mL/sec). The mean prostate volume as measured by transrectal ultrasound was 36.3 mL (±20.1 mL). Prostate volume was ≤20 mL in 16% of patients, ≥50 mL in 18% of patients and between 21 and 49 mL in 66% of patients.
The primary endpoint was a composite measure of the first occurrence of any of the following five outcomes: a ≥4 point confirmed increase from baseline in symptom score, acute urinary retention, BPH-related renal insufficiency (creatinine rise), recurrent urinary tract infections or urosepsis, or incontinence. Compared to placebo, treatment with PROSCAR, doxazosin, or combination therapy resulted in a reduction in the risk of experiencing one of these five outcome events by 34% (p=0.002), 39% (p<0.001), and 67% (p<0.001), respectively. Combination therapy resulted in a significant reduction in the risk of the primary endpoint compared to treatment with PROSCAR alone (49%; p≤0.001) or doxazosin alone (46%; p≤0.001). (See Table 6.)
Table 6: Count and Percent Incidence of Primary Outcome Events by Treatment Group in MTOPS Treatment Group Placebo Doxazosin Finasteride Combination Total N=737 N=756 N=768 N=786 N=3047 Event N (%) N (%) N (%) N (%) N (%) AUA 4-point rise 100 (13.6) 59 (7.8) 74 (9.6) 41 (5.2) 274 (9.0) Acute urinary retention 18 (2.4) 13 (1.7) 6 (0.8) 4 (0.5) 41 (1.3) Incontinence 8 (1.1) 11 (1.5) 9 (1.2) 3 (0.4) 31 (1.0) Recurrent UTI/urosepsis 2 (0.3) 2 (0.3) 0 (0.0) 1 (0.1) 5 (0.2) Creatinine rise 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) Total Events 128 (17.4) 85 (11.2) 89 (11.6) 49 (6.2) 351 (11.5) The majority of the events (274 out of 351; 78%) was a confirmed ≥4 point increase in symptom score, referred to as symptom score progression. The risk of symptom score progression was reduced by 30% (p=0.016), 46% (p<0.001), and 64% (p<0.001) in patients treated with PROSCAR, doxazosin, or the combination, respectively, compared to patients treated with placebo (see Figure 5). Combination therapy significantly reduced the risk of symptom score progression compared to the effect of PROSCAR alone (p<0.001) and compared to doxazosin alone (p=0.037).
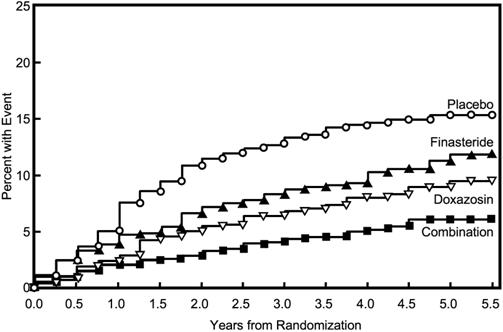
Figure 5: Cumulative Incidence of a 4-Point Rise in AUA Symptom Score by Treatment Group
Treatment with PROSCAR, doxazosin or the combination of PROSCAR with doxazosin, reduced the mean symptom score from baseline at year 4. Table 7 provides the mean change from baseline for AUA symptom score by treatment group for patients who remained on therapy for four years.
Table 7: Change From Baseline in AUA Symptom Score by Treatment Group at Year 4 in MTOPS Placebo
N=534Doxazosin
N=582Finasteride
N=565Combination
N=598Baseline Mean (SD) 16.8 (6.0) 17.0 (5.9) 17.1 (6.0) 16.8 (5.8) Mean Change
AUA Symptom Score (SD)-4.9 (5.8) -6.6 (6.1) -5.6 (5.9) -7.4 (6.3) Comparison to
Placebo (95% CI)-1.8
(-2.5, -1.1)-0.7
(-1.4, 0.0)-2.5
(-3.2, -1.8)Comparison to
Doxazosin alone (95% CI)-0.7
(-1.4, 0.0)Comparison to
Finasteride alone (95% CI)-1.8
(-2.5, -1.1)The results of MTOPS are consistent with the findings of the 4-year, placebo-controlled study PLESS [see Clinical Studies (14.1)] in that treatment with PROSCAR reduces the risk of acute urinary retention and the need for BPH-related surgery. In MTOPS, the risk of developing acute urinary retention was reduced by 67% in patients treated with PROSCAR compared to patients treated with placebo (0.8% for PROSCAR and 2.4% for placebo). Also, the risk of requiring BPH-related invasive therapy was reduced by 64% in patients treated with PROSCAR compared to patients treated with placebo (2.0% for PROSCAR and 5.4% for placebo).
14.3 Summary of Clinical Studies
The data from these studies, showing improvement in BPH-related symptoms, reduction in treatment failure (BPH-related urological events), increased maximum urinary flow rates, and decreasing prostate volume, suggest that PROSCAR arrests the disease process of BPH in men with an enlarged prostate.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
No. 3094 — PROSCAR tablets 5 mg are blue, modified apple-shaped, film-coated tablets, with the code MSD 72 on one side and PROSCAR on the other. They are supplied as follows:
NDC: 0006-0072-31 unit of use bottles of 30
NDC: 0006-0072-58 unit of use bottles of 100.
Storage and Handling
Store at room temperatures below 30°C (86°F). Protect from light and keep container tightly closed.
Women should not handle crushed or broken PROSCAR tablets when they are pregnant or may potentially be pregnant because of the possibility of absorption of finasteride and the subsequent potential risk to a male fetus [see Warnings and Precautions (5.3), Use in Specific Populations (8.1) and Patient Counseling Information (17.2)].
-
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling (Patient Information).
17.1 Increased Risk of High-Grade Prostate Cancer
Patients should be informed that there was an increase in high-grade prostate cancer in men treated with 5α-reductase inhibitors indicated for BPH treatment, including PROSCAR, compared to those treated with placebo in studies looking at the use of these drugs to prevent prostate cancer [see Indications and Usage (1.3), Warnings and Precautions (5.2), and Adverse Reactions (6.1)].
17.2 Exposure of Women — Risk to Male Fetus
Physicians should inform patients that women who are pregnant or may potentially be pregnant should not handle crushed or broken PROSCAR tablets because of the possibility of absorption of finasteride and the subsequent potential risk to the male fetus. PROSCAR tablets are coated and will prevent contact with the active ingredient during normal handling, provided that the tablets have not been broken or crushed. If a woman who is pregnant or may potentially be pregnant comes in contact with crushed or broken PROSCAR tablets, the contact area should be washed immediately with soap and water [see Contraindications (4), Warnings and Precautions (5.3), Use in Specific Populations (8.1) and How Supplied/Storage and Handling (16)].
17.3 Additional Instructions
Physicians should inform patients that the volume of ejaculate may be decreased in some patients during treatment with PROSCAR. This decrease does not appear to interfere with normal sexual function. However, impotence and decreased libido may occur in patients treated with PROSCAR [see Adverse Reactions (6.1)].
Physicians should instruct their patients to promptly report any changes in their breasts such as lumps, pain or nipple discharge. Breast changes including breast enlargement, tenderness and neoplasm have been reported [see Adverse Reactions (6.1)].
Physicians should instruct their patients to read the patient package insert before starting therapy with PROSCAR and to reread it each time the prescription is renewed so that they are aware of current information for patients regarding PROSCAR.
-
SPL UNCLASSIFIED SECTION
Dist. by: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC., Whitehouse Station, NJ 08889, USAFor patent information: www.merck.com/product/patent/home.html
Copyright © 1992, 1995, 1998, 2011 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved.uspi-mk0906-5t-1309r011
-
PATIENT PACKAGE INSERT
PROSCAR® (finasteride) Tablets
Patient Information about
PROSCAR® (Prahs-car)
Generic name: finasteride
(fin-AS-tur-eyed)PROSCAR is for use by men only.
Please read this leaflet before you start taking PROSCAR. Also, read it each time you renew your prescription, just in case anything has changed. Remember, this leaflet does not take the place of careful discussions with your doctor. You and your doctor should discuss PROSCAR when you start taking your medication and at regular checkups.
What is PROSCAR?
PROSCAR is a medication used to treat symptoms of benign prostatic hyperplasia (BPH) in men with an enlarged prostate. PROSCAR may also be used to reduce the risk of a sudden inability to pass urine and the need for surgery related to BPH in men with an enlarged prostate.
PROSCAR may be prescribed along with another medicine, an alpha-blocker called doxazosin, to help you better manage your BPH symptoms.
Who should NOT take PROSCAR?
PROSCAR is for use by MEN only.
Do Not Take PROSCAR if you are:
- a woman who is pregnant or may potentially be pregnant. PROSCAR may harm your unborn baby. Do not touch or handle crushed or broken PROSCAR tablets (see "A warning about PROSCAR and pregnancy").
- allergic to finasteride or any of the ingredients in PROSCAR. See the end of this leaflet for a complete list of ingredients in PROSCAR.
A warning about PROSCAR and pregnancy:
Women who are or may potentially be pregnant must not use PROSCAR. They should also not handle crushed or broken tablets of PROSCAR. PROSCAR tablets are coated and will prevent contact with the active ingredient during normal handling, provided that the tablets are not broken or crushed.
If a woman who is pregnant with a male baby absorbs the active ingredient in PROSCAR after oral use or through the skin, it may cause the male baby to be born with abnormalities of the sex organs. If a woman who is pregnant comes into contact with the active ingredient in PROSCAR, a doctor should be consulted.
How should I take PROSCAR?
Follow your doctor's instruction.
- Take one tablet by mouth each day. To avoid forgetting to take PROSCAR, you can take it at the same time every day.
- If you forget to take PROSCAR, do not take an extra tablet. Just take the next tablet as usual.
- You may take PROSCAR with or without food.
- Do not share PROSCAR with anyone else; it was prescribed only for you.
What are the possible side effects of PROSCAR?
PROSCAR may increase the chance of a more serious form of prostate cancer.
The most common side effects of PROSCAR include:
- trouble getting or keeping an erection (impotence)
- decrease in sex drive
- decreased volume of ejaculate
- ejaculation disorders
- enlarged or painful breast. You should promptly report to your doctor any changes in your breasts such as lumps, pain or nipple discharge.
The following have been reported in general use with PROSCAR and/or finasteride at lower doses:
- allergic reactions, including rash, itching, hives, and swelling of the lips, tongue, throat, and face
- rarely, some men may have testicular pain
- trouble getting or keeping an erection that continued after stopping the medication
- problems with ejaculation that continued after stopping the medication
- male infertility and/or poor quality of semen. Improvement in the quality of semen has been reported after stopping the medication.
- depression
- decrease in sex drive that continued after stopping the medication
- in rare cases, male breast cancer has been reported.
You should discuss side effects with your doctor before taking PROSCAR and anytime you think you are having a side effect. These are not all the possible side effects with PROSCAR. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at: 1-800-FDA-1088.
What you need to know while taking PROSCAR:
- You should see your doctor regularly while taking PROSCAR. Follow your doctor's advice about when to have these checkups.
- Checking for prostate cancer. Your doctor has prescribed PROSCAR for BPH and not for treatment of prostate cancer — but a man can have BPH and prostate cancer at the same time. Your doctor may continue checking for prostate cancer while you take PROSCAR.
- About Prostate-Specific Antigen (PSA). Your doctor may have done a blood test called PSA for the screening of prostate cancer. Because PROSCAR decreases PSA levels, you should tell your doctor(s) that you are taking PROSCAR. Changes in PSA levels will need to be evaluated by your doctor(s). Any increase in follow-up PSA levels from their lowest point may signal the presence of prostate cancer and should be evaluated, even if the test results are still within the normal range. You should also tell your doctor if you have not been taking PROSCAR as prescribed because this may affect the PSA test results. For more information, talk to your doctor.
How should I store PROSCAR?
- Store PROSCAR tablets in a dry place at room temperature.
- Keep PROSCAR in the original container and keep the container closed.
PROSCAR tablets are coated and will prevent contact with the active ingredient during normal handling, provided that the tablets are not broken or crushed.
Keep PROSCAR and all medications out of the reach of children.
Do not give your PROSCAR tablets to anyone else. It has been prescribed only for you.
For more information call 1-800-622-4477.
What are the ingredients in PROSCAR?
Active ingredients: finasteride
Inactive ingredients: hydrous lactose, microcrystalline cellulose, pregelatinized starch, sodium starch glycolate, hydroxypropyl cellulose LF, hydroxypropyl methylcellulose, titanium dioxide, magnesium stearate, talc, docusate sodium, FD&C Blue 2 aluminum lake and yellow iron oxide.
What is BPH?
BPH is an enlargement of the prostate gland. The prostate is located below the bladder. As the prostate enlarges, it may slowly restrict the flow of urine. This can lead to symptoms such as:
- a weak or interrupted urinary stream
- a feeling that you cannot empty your bladder completely
- a feeling of delay or hesitation when you start to urinate
- a need to urinate often, especially at night
- a feeling that you must urinate right away.
In some men, BPH can lead to serious problems, including urinary tract infections, a sudden inability to pass urine (acute urinary retention), as well as the need for surgery.
What PROSCAR does:
PROSCAR lowers levels of a hormone called DHT (dihydrotestosterone), which is a cause of prostate growth. Lowering DHT leads to shrinkage of the enlarged prostate gland in most men. This can lead to gradual improvement in urine flow and symptoms over the next several months. PROSCAR will help reduce the risk of developing a sudden inability to pass urine and the need for surgery related to an enlarged prostate. However, since each case of BPH is different, you should know that:
- Even though the prostate shrinks, you may NOT notice an improvement in urine flow or symptoms.
- You may need to take PROSCAR for six (6) months or more to see whether it improves your symptoms.
- Therapy with PROSCAR may reduce your risk for a sudden inability to pass urine and the need for surgery for an enlarged prostate.
-
SPL UNCLASSIFIED SECTION
Dist. by: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC., Whitehouse Station, NJ 08889, USAFor patent information: www.merck.com/product/patent/home.html
Copyright © 1992, 1995, 1998, 2011 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved.Revised: 09/2013
usppi-mk0906-5t-1309r011
-
PRINCIPAL DISPLAY PANEL - 5 mg Tablet Bottle Label
NDC: 0006-0072-31
Proscar®
(finasteride) Tablets5 mg
WARNING: PROSCAR® (finasteride) should
not be used by women or children.
Women who are or may potentially be
pregnant must not use PROSCAR. They
should also not handle crushed or broken
tablets of PROSCAR. (See Package Insert.)Rx only
30 Tablets
-
INGREDIENTS AND APPEARANCE
PROSCAR
finasteride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0006-0072 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FINASTERIDE (UNII: 57GNO57U7G) (FINASTERIDE - UNII:57GNO57U7G) FINASTERIDE 5 mg Inactive Ingredients Ingredient Name Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) ALUMINUM OXIDE (UNII: LMI26O6933) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color BLUE Score no score Shape FREEFORM (modified apple shaped) Size 7mm Flavor Imprint Code MSD;72;PROSCAR Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0006-0072-31 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/19/1992 2 NDC: 0006-0072-58 100 in 1 BOTTLE; Type 0: Not a Combination Product 06/19/1992 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020180 06/19/1992 Labeler - Merck Sharp & Dohme Corp. (001317601)
Trademark Results [PROSCAR]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PROSCAR 73757633 1546049 Live/Registered |
Merck & Co., Inc. 1988-10-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.