OLOPATADINE HYDROCHLORIDE solution
olopatadine hydrochloride by
Drug Labeling and Warnings
olopatadine hydrochloride by is a Otc medication manufactured, distributed, or labeled by Rising Pharma Holdings, Inc., Micro Labs Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT(S)
- PURPOSE
- USE(S)
- WARNINGS
- DO NOT USE
- WHEN USING THIS PRODUCT
- STOP USE AND ASK DOCTOR IF
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
▪ adults and children 2 years of age and older:
▪ put 1 drop in the affected eye(s) twice daily, every 6 to 8 hours, no more than twice per day
▪ if using other ophthalmic products while using this product, wait at least 5 minutes between each product
▪ replace cap after each use
▪ children under 2 years of age:
consult a doctor - Other information
- Inactive ingredients
- Questions?
-
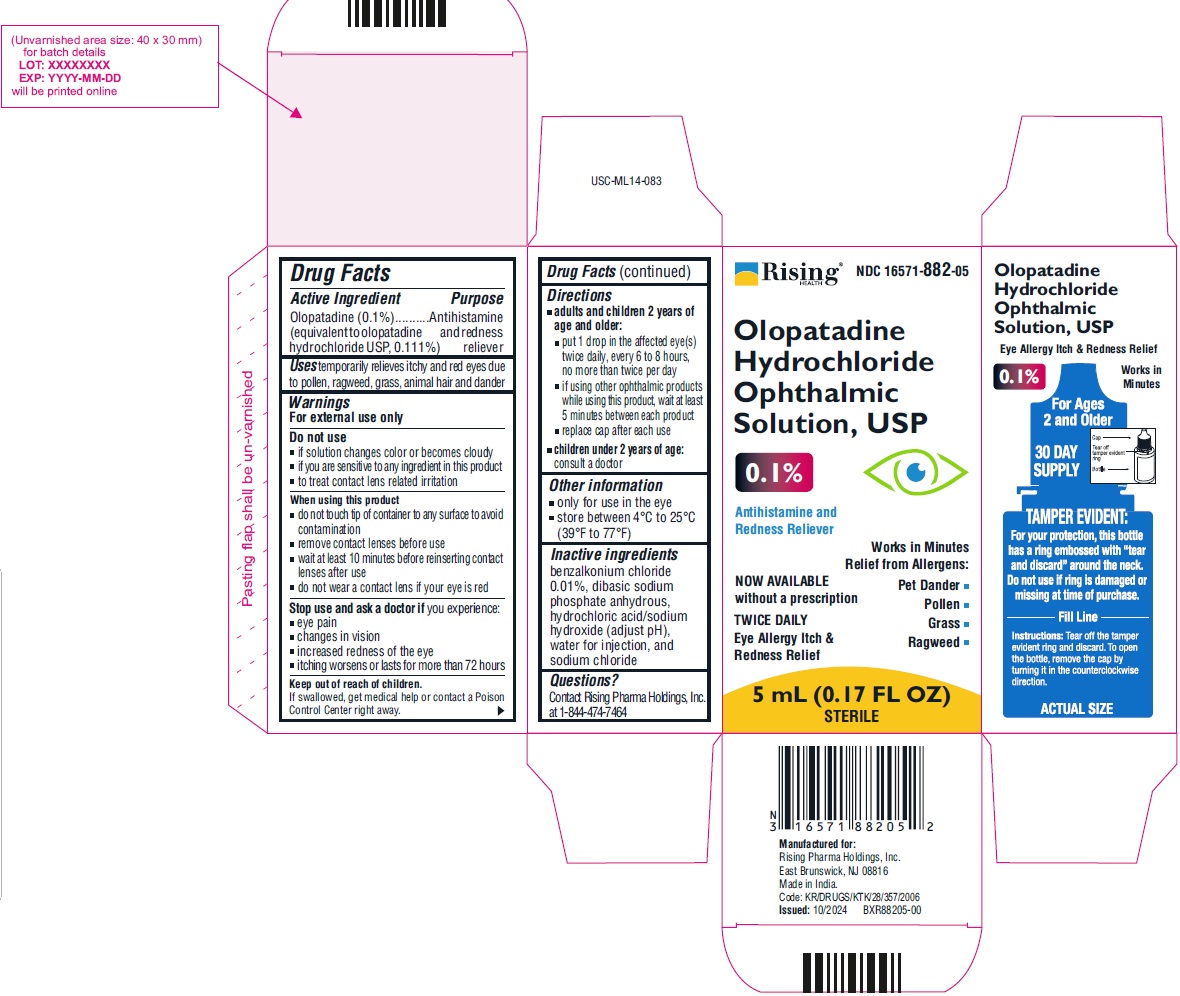
PRINCIPAL DISPLAY PANEL
Rising NDC: 16571-882-05
Olopatadine Hydrochloride Ophthalmic Solution, USP 0.1%Antihistamine and Redness Reliever
NOW AVAILABLE without a prescription
TWICE DAILYEye Allergy Itch & Redness Relief
Works in Minutes Relief from Allergens:
Pet Dander
Grass
Pollen
Ragweed5 mL (0.17 FL OZ) STERILE
Rising NDC 16571-882-05
Olopatadine Hydrochloride Ophthalmic Solution, USP
0.1%
Antihistamine and Redness Reliever
Eye Allergy Itch & Redness Relief
TWICE DAILY
5 mL (0.17 FL OZ)
STERILE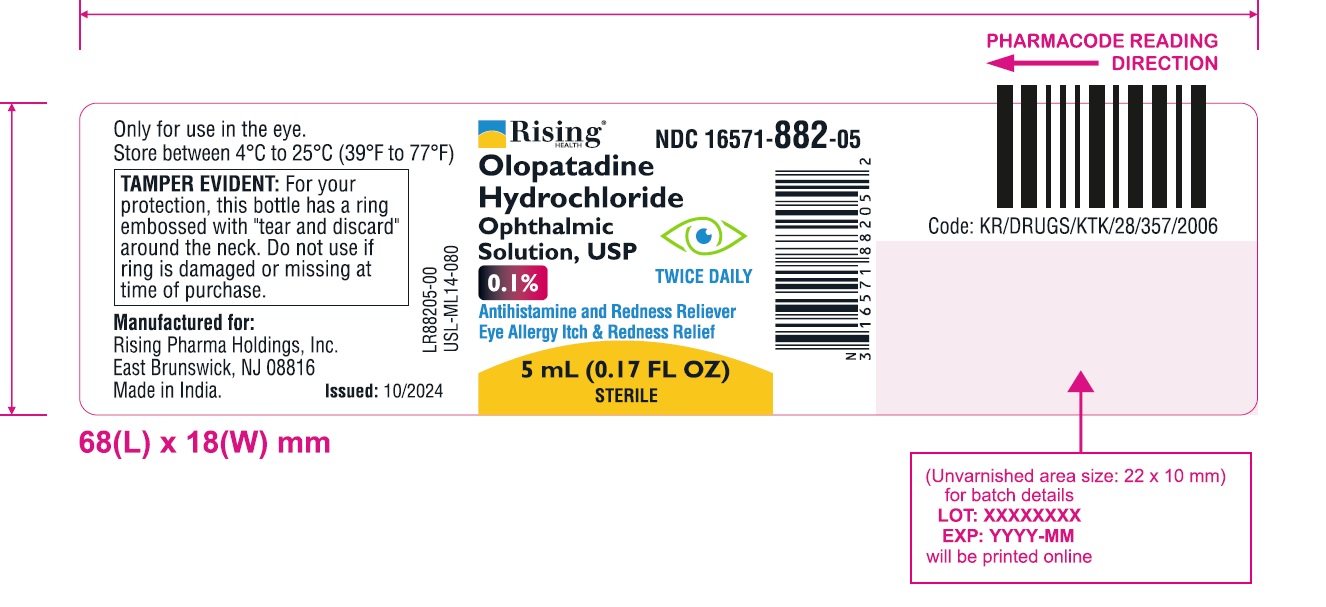
-
INGREDIENTS AND APPEARANCE
OLOPATADINE HYDROCHLORIDE
olopatadine hydrochloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 16571-882 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OLOPATADINE HYDROCHLORIDE (UNII: 2XG66W44KF) (OLOPATADINE - UNII:D27V6190PM) OLOPATADINE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 16571-882-05 1 in 1 CARTON 05/01/2025 1 5 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204392 05/01/2025 Labeler - Rising Pharma Holdings, Inc. (116880195) Establishment Name Address ID/FEI Business Operations Micro Labs Limited 677600482 ANALYSIS(16571-882) , LABEL(16571-882) , MANUFACTURE(16571-882) , PACK(16571-882)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.