YCANTH- cantharidin solution
YCANTH by
Drug Labeling and Warnings
YCANTH by is a Prescription medication manufactured, distributed, or labeled by Verrica Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use YCANTH safely and effectively. See full prescribing information for YCANTH.
YCANTH® (cantharidin) topical solution
Initial U.S. Approval: 2023INDICATIONS AND USAGE
YCANTH is indicated for the topical treatment of molluscum contagiosum in adult and pediatric patients 2 years of age and older. (1)
DOSAGE AND ADMINISTRATION
- All healthcare professionals should receive instruction and training prior to preparation and administration of YCANTH. (2.1)
- For topical use only. Not for oral, mucosal, or ophthalmic use. (2.1)
- Apply a single application directly to each lesion every 3 weeks as needed. (2.2)
- Do not use more than two applicators during a single treatment session. (2.2)
- Remove with soap and water 24 hours after treatment. (2.2)
- For additional instructions on preparation and administration of YCANTH, see Full Prescribing Information. (2.1, 2.2, 2.3)
DOSAGE FORMS AND STRENGTHS
Topical solution: 0.7% cantharidin. (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
- Toxicities Associated with Inappropriate Administration: Life threatening or fatal toxicities can occur if administered orally. Avoid contact with the treatment area, including oral contact, after treatment. Ocular toxicity can occur if YCANTH comes in contact with eyes. If YCANTH gets in eyes, flush eyes with water for at least 15 minutes. (5.1)
- Local Skin Reactions: Reactions at the application site have included vesiculation, pruritus, pain, discoloration, and erythema. Avoid application near eyes and mucosal tissue, and to healthy skin. If YCANTH contacts any unintended surface, or healthy skin, immediately remove. If severe local skin reactions occur, remove prior to 24 hours after treatment. (5.2)
- Flammability: YCANTH is flammable, even after drying. Avoid fire, flame or smoking near lesion(s) during treatment and after application until removed. (5.3)
ADVERSE REACTIONS
Most common (incidence ≥1%) adverse reactions are the following local skin reactions at the application site: vesiculation, pain, pruritus, scabbing, erythema, discoloration, application site dryness, edema, and erosion. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Verrica Pharmaceuticals Inc. at 1-877-837-7422 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. Local skin reactions are expected and should be reported if they are severe.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 10/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Dosage and Administration Overview
2.3 Dosage and Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Toxicities Associated with Inappropriate Administration
5.2 Local Skin Reactions
5.3 Flammability
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/ STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
- All healthcare professionals should receive instruction and training prior to preparation and administration of YCANTH.
- YCANTH is for topical use only.
- YCANTH is not for oral, mucosal, or ophthalmic use. Avoid contact with the treatment area, including oral contact, after YCANTH treatment. Do not apply YCANTH near the eyes [see Warnings and Precautions (5.1), Overdosage (10)].
- If YCANTH contacts any unintended surface, including healthy skin, immediately remove by wiping with a cotton swab or gauze. Avoid other topical products on treated areas until 24 hours after YCANTH treatment or until washing [see Warnings and Precautions (5.2)].
- Avoid fire, flame or smoking near lesion(s) during treatment [see Warnings and Precautions (5.3)].
2.2 Dosage and Administration Overview
- Regarding YCANTH dosage and administration:
- Use nitrile or vinyl gloves and eye protection during preparation and administration.
- Apply topically as a single application to cover each lesion. Use no more than two YCANTH applicators during a single treatment session.
- Remove with soap and water 24 hours after treatment. Administer YCANTH every 3 weeks as needed.
- Do not cover any treated lesions with bandages.
- If severe blistering, severe pain, or other severe adverse reactions occur, remove YCANTH with soap and water prior to 24 hours after treatment [see Warnings and Precautions (5.2)].
- Regarding use of the YCANTH applicator and break tool:
- Do not reuse the applicator. The applicator is for a single treatment session only.
- Do not attempt to use a clogged applicator.
- Do not cut or modify the applicator in any way; doing so could reduce dispensing control.
- Do not remove the applicator cap prior to breaking the glass ampule.
- If any damage or leaks are observed on the applicator, applicators should be discarded in a sharps container and handled in accordance with accepted medical practice and applicable law. The YCANTH Break Tool should be managed as solid waste and placed in plastic recycling containers or the general trash.
2.3 Dosage and Administration Instructions
Instructions for preparing the YCANTH Applicator and administering the YCANTH Solution are presented below.
YCANTH includes the following components [see How Supplied/Storage and Handling (16.1)]:
Outer Protective Tube 
The YCANTH Applicator 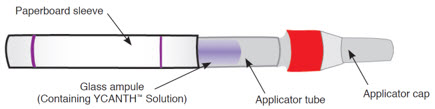
The YCANTH Break Tool 
Step 1. Put on Personal Protective Equipment (PPE) - Put on gloves and eye protection.

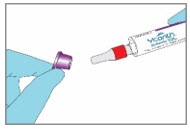
Step 2. Remove the YCANTH Applicator from Outer Protective Tube - Use pull ring to remove purple end cap out of white outer protective tube.
- DO NOT remove the applicator cap.
- Slide the YCANTH Applicator out of the outer protective tube.
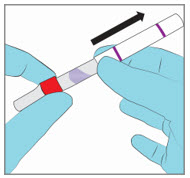
Step 3. Remove the Paperboard Sleeve - Completely remove the white paperboard sleeve so that the glass ampule is visible.
- DO NOT remove the applicator cap prior to breaking the glass ampule.
Step 4. Inspect for Damage - Inspect the YCANTH Applicator for the following types of damage:
Broken glass ampule

Cut or cracked applicator tube

No damage (for reference)

- If any damage is observed, dispose of the YCANTH applicators in a sharps container and handle in accordance with accepted medical practice and applicable law.
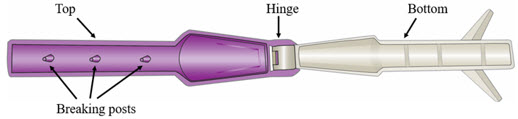
Step 5. Break Ampule using the YCANTH Break Tool - Set the YCANTH Break Tool on a horizontal surface.
- Ensure the applicator cap is in place.
- Place the YCANTH Applicator in the YCANTH Break Tool with the cap pointing toward the hinge.
- Press down firmly on the YCANTH Break Tool until a snap is heard.
- Open the YCANTH Break Tool and remove the YCANTH Applicator.
- If any leaks are observed, applicators should be discarded in a sharps container and handled in accordance with accepted medical practice and applicable law.

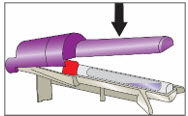
Step 6. Tap YCANTH Applicator to move YCANTH Solution - Gently tap the capped end of the YCANTH Applicator on a horizontal surface for approximately 10 seconds or until the YCANTH Solution has collected at the bottom of the applicator tube.
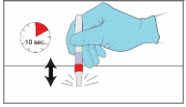
Step 7. Test the YCANTH Applicator - Remove the applicator cap.
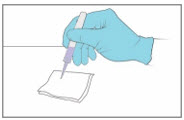
- Gently squeeze the applicator tube to apply a droplet to a paper towel or gauze to confirm the YCANTH Applicator is working properly.
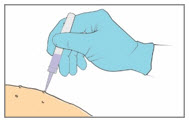
Step 8. Apply the YCANTH Solution - Apply a small droplet of the YCANTH Solution onto a molluscum lesion.
- Use the applicator tip to spread the solution to cover the entire lesion.
- Repeat the application until all lesions have been treated.
- If YCANTH Solution contacts any unintended surface, including healthy skin, immediately remove by wiping with a cotton swab or gauze.
- DO NOT attempt to use a clogged YCANTH Applicator.
Step 9. Allow YCANTH Solution to Dry - Allow the YCANTH Solution to completely dry (up to 5 minutes) before contacting healthy skin to avoid transference.
- DO NOT cover any treated lesions with bandages.
Step 10. Dispose of the YCANTH Applicator - Applicators should be discarded in a sharps container and handled in accordance with accepted medical practice and applicable law.
- DO NOT reuse the YCANTH Applicator.
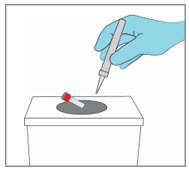
YCANTH Break Tool Maintenance
- Wipe the surfaces of the YCANTH Break Tool with 70% isopropyl alcohol following each use.
- Inspect the YCANTH Break Tool for damage (e.g., cracks in plastic, missing or broken breaking posts) and function (e.g., hinge) prior to each use.
- After 12 uses or if any damage is observed, the YCANTH Break Tool should be managed as solid waste and placed in plastic recycling containers or the general trash.
- If needed, contact Verrica Pharmaceuticals Inc. at 1-973-298-1390 to request additional YCANTH Break Tools.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Toxicities Associated with Inappropriate Administration
YCANTH is for topical use only. YCANTH is not for oral, mucosal, or ophthalmic use.
Life threatening or fatal toxicities can occur if YCANTH is administered orally [see Overdosage (10)]. Adverse reactions to oral ingestion of cantharidin have included renal failure, blistering and severe damage to the gastrointestinal tract, coagulopathy, seizures, and flaccid paralysis. Advise patients and/or caregivers to avoid oral contact and to avoid touching lesions after YCANTH treatment and to seek medical attention immediately if YCANTH is accidently ingested.
Ocular toxicity can occur if YCANTH comes in contact with the eyes. Adverse reactions from contact of YCANTH with the eyes can include corneal necrosis, ocular perforation, and deep ocular injuries. Do not apply YCANTH near or to the eyes. If YCANTH comes in contact with the eyes, flush eyes with water for at least 15 minutes and seek medical attention immediately.
5.2 Local Skin Reactions
YCANTH is a vesicant. Local skin reactions at the application site were observed in 97% of subjects treated with YCANTH during clinical trials. Local skin reactions included vesiculation, pruritus, pain, discoloration, and erythema [see Adverse Reactions (6.1)].
Avoid application near the eyes and mucosal tissues, and to adjacent healthy skin. If YCANTH contacts any unintended surface, including healthy skin, immediately remove by wiping with a cotton swab or gauze.
Avoid other topical products (e.g. creams, lotions, or sunscreen) on treated areas until 24 hours after YCANTH treatment or until washing. Application of other topical products could spread YCANTH and cause blistering or other adverse reactions to healthy skin.
If severe blistering, severe pain or other severe adverse reactions occur, remove YCANTH prior to the recommended 24 hours after administration by washing with soap and water.
-
6 ADVERSE REACTIONS
The following adverse reactions are described elsewhere in the labeling:
- Local Skin Reactions [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
YCANTH was studied in two randomized, double-blind, placebo-controlled phase 3 trials, Trial 1 (NCT03377790) and Trial 2 (NCT03377803) (n = 266, and n = 262, respectively) in subjects with molluscum contagiosum. Most patients received a single 24-hour dermal administration of YCANTH or vehicle for each lesion every 3 weeks for up to 4 treatments. YCANTH Solution or vehicle were removed prior to the 24-hour timepoint in 109/311 (35%) subjects treated with YCANTH Solution and 46/216 (21%) subjects treated with vehicle due to treatment-emergent adverse events.
Table 1 presents the percentage of subjects with selected adverse reactions (incidence ≥ 1%) by the most severe grade reported during Trial 1 and Trial 2. Adverse reactions were primarily local skin reactions at the application site. Local skin reactions at the application site were observed in 97% of subjects treated with YCANTH during both trials.
Table 1. Percentage of Subjects with Selected Adverse Reactions (Incidence ≥1%) by Severity in Trial 1 and Trial 2 (Safety Population) YCANTH
N=311Vehicle
N=216Preferred Term Name Mild Moderate Severe Mild Moderate Severe Application site vesicles 60% 32% 4% 27% 2% 0% Application site pain and pain 41% 20% 2% 16% 1% 0% Application site pruritus and pruritus 47% 8% 1% 30% 7% 0% Application site scab and scab 39% 9% 0% 20% 1% 0% Application site erythema and erythema 24% 21% <1% 20% 7% 0% Application site discoloration 28% 4% <1% 12% 1% 0% Application site dryness 19% 2% 0% 14% 1% 0% Application site edema 7% 3% 0% 3% 1% 0% Application site erosion 6% 1% 0% 1% 0% 0% Contact dermatitis 0% 1% 0% 0% 0% 0% There were no serious adverse reactions reported in the two controlled trials.
The discontinuation rate due to an adverse reaction was 2.3% among subjects treated with YCANTH and 0.5% among subjects treated with vehicle.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data with use of YCANTH in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Animal reproduction studies were not conducted with cantharidin. Given that systemic exposure to cantharidin following topical administration is low, maternal use is not expected to result in fetal exposure to the drug [see Clinical Pharmacology (12.3)].
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
Avoid application of YCANTH topical solution to areas with increased risk for potential ingestion by or ocular exposure to the breastfeeding child. There are no data on the presence of cantharidin in either human or animal milk, or the effects on the breastfed infant or on milk production. Breastfeeding is not expected to result in exposure of the child to the drug due to the low systemic absorption of YCANTH following topical administration [see Clinical Pharmacology (12.3)]. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for YCANTH topical solution and any potential adverse effects on the breastfeeding child from YCANTH topical solution or from the underlying maternal condition.
8.4 Pediatric Use
Risk Summary
The safety and effectiveness of YCANTH for the treatment of molluscum contagiosum have been established in pediatric patients aged 2 years and older. The use of YCANTH in pediatric patients is supported by results from adequate and well-controlled trials in patients 2 years of age and older; although the safety and efficacy of drug use for longer than 12 weeks has not been established [see Clinical Studies (14)].
The safety and efficacy in pediatric patients below the age of 2 years have not been established.
-
10 OVERDOSAGE
Oral ingestion of cantharidin has resulted in renal failure, blistering and severe damage to the gastrointestinal tract, coagulopathy, seizures, and flaccid paralysis.
In the event that YCANTH topical solution is ingested, patients should seek medical attention immediately and contact a Poison Control Center (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations.
-
11 DESCRIPTION
YCANTH (cantharidin) topical solution is a light violet to dark purple, slightly viscous liquid for topical administration.
Each mL of YCANTH topical solution contains 7 mg of active ingredient cantharidin (0.7%), a lipophilic compound. Cantharidin is a white to off-white solid at room temperature and is only very slightly soluble in water.
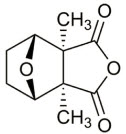
The chemical name for cantharidin is 1,2-dimethyl-3,6-epoxyperhydrophthalic anhydride. The molecular weight is 196.20 g/mol and the molecular formula is C10H12O4. The structural formula for cantharidin is represented below:
The inactive ingredients of YCANTH topical solution are acetone, camphor, castor oil, denatonium benzoate, ethanol (32%), gentian violet, hydroxypropyl cellulose, and nitrocellulose. The inactive ingredient denatonium benzoate is an oral deterrent to help mitigate the risk of accidental ingestion.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of cantharidin in the treatment of molluscum contagiosum is unknown.
12.2 Pharmacodynamics
Cantharidin is a vesicant. The pharmacodynamics of cantharidin in the treatment of molluscum contagiosum are unknown.
12.3 Pharmacokinetics
Pharmacokinetics of cantharidin were assessed in 16 subjects aged 2 years and older with at least 21 molluscum contagiosum lesions at baseline. Subjects were administered a single, dermal application of cantharidin solution to each lesion. The average number of lesions treated for subjects in the exposure treatment group was 47.4 (median was 35.0).
Cantharidin was not detectable in 15 out of 16 subjects. Only one subject had a detectable cantharidin plasma level of 3.391 ng/mL at the 2-hour post-dose.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with cantharidin.
Cantharidin was not mutagenic in a bacteria reverse mutation (Ames) assay. An in vitro chromosomal aberration assay in human lymphocytes with cantharidin was inconclusive. Cantharidin was positive in an in vitro micronucleus assay in TK6 cells, primarily through an aneugenic mechanism.
The effects of cantharidin on fertility have not been evaluated.
-
14 CLINICAL STUDIES
In two double-blind, randomized, placebo-controlled trials (Trial 1 [NCT03377790] and Trial 2 [NCT03377803]), 528 subjects ages 2 years and older with molluscum contagiosum were randomized by household to treatment with either YCANTH or vehicle. Subjects ranged from 2 to 60 years of age, with a median age of 6. 51% of subjects were male and 91% were Caucasian. The baseline lesion count among subjects ranged from 1 to 184, with a mean of 23 and 19 lesions among subjects in Trial 1 and Trial 2, respectively.
Subjects' lesions were treated with either YCANTH or vehicle at intervals of approximately 21 days until complete clearance of the lesion or for a maximum of 4 applications (on Days 1, 21, 42, and 63). Study drug solution was applied and left on the lesions for approximately 24 hours before the lesions were washed with soap and water.
A healthcare professional who was blinded to the treatment group counted the number of lesions at each visit. The primary efficacy endpoint was the proportion of patients achieving complete clearance of all treated molluscum contagiosum lesions by Day 84. The secondary efficacy endpoints were the proportions of patients achieving complete clearance of all treated molluscum contagiosum lesions at Day 63, Day 42, and Day 21. Table 2 presents the efficacy results for Trial 1 and Trial 2.
Table 2. Percentage of Subjects Exhibiting Complete Clearance of Treatable Molluscum Contagiosum Lesions in Trial 1 and Trial 2 (Intent-to-Treat Population) Trial 1 Trial 2 YCANTH
N = 160Vehicle
N = 106Treatment Difference
(95% CI)*YCANTH
N = 150Vehicle
N = 112Treatment Difference
(95% CI)*CI = confidence interval. - * Treatment difference and 95% CI based on Generalized Estimating Equations (GEE) model for logistic regression with an exchangeable working correlation structure, a factor for treatment, and repeated measurements allowed for a household. Subjects with missing data are imputed as non-responders.
Day 84 46% 18% 29%
(19%, 38%)54% 13% 40%
(30%, 51%)Day 63 32% 17% 15%
(4%, 25%)28% 5% 23%
(15%, 32%)Day 42 21% 9% 10%
(2%, 19%)13% 4% 9%
(3%, 16%)Day 21 11% 4% 8%
(2%, 14%)5% 2% 3%
(-1%, 8%) -
16 HOW SUPPLIED/ STORAGE AND HANDLING
16.1 How Supplied
YCANTH topical solution is supplied in a sealed glass ampule contained within a single-use applicator and enclosed in a protective paperboard sleeve. Each ampule of YCANTH contains approximately 0.45 mL of 0.7% cantharidin solution. Each mL of YCANTH contains 7 mg cantharidin (0.7%). A YCANTH Break Tool is co-packaged as 2 units per each 6 count and 12 count carton of applicators and 1 unit per each 1 count carton with 1 applicator.
A listing of the available carton packages is provided in Table 3.
Table 3. Available Carton Configurations for YCANTH Dosage Strength Single-Use Applicator Single Applicator NDC (Each) Number of YCANTH Applicators (Each) per Carton YCANTH Break Tool Part Reference Number Number of YCANTH Break Tools (Each) per Carton NDC 0.7%
(7 mg/mL)1 each 71349-070-01 1 71349-000-01 1 71349-070-11 6 71349-000-01 2 71349-070-06 12 71349-000-01 2 71349-070-12 Individual YCANTH Break Tools are available separately (Part Reference Number 71349-000-01).
To request additional YCANTH Break Tools, contact Verrica Pharmaceuticals at 1-973-298-1390.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient and/or caregivers to read the FDA-approved patient labeling (Patient Information).
Toxicities Associated with Inappropriate Administration
Inform patients and/or caregivers that YCANTH can be fatal if ingested orally. Instruct patients and/or caregivers to avoid oral contact and to avoid touching lesions after YCANTH treatment. If YCANTH is accidentally ingested, inform patients and/or caregivers to seek medical attention immediately.
Advise patients and/or caregivers that severe eye injury can occur if YCANTH gets into the eyes. If YCANTH gets in the eyes, advise patients and/or caregivers to flush eyes with water for at least 15 minutes and seek medical attention immediately [see Warnings and Precautions (5.1)].
Local Skin Reactions
Inform patients and/or caregivers that treatment with YCANTH can lead to local skin reactions [see Adverse Reactions (6.1)].
Advise patients and/or caregivers not to use other topical products (e.g. creams, lotions, or sunscreen) until 24 hours after YCANTH treatment or until washing. Advise patients and/or caregivers to remove YCANTH with soap and water prior to 24 hours after treatment and contact the healthcare provider if severe blistering, severe pain or other severe adverse reactions occur [see Warnings and Precautions (5.2)].
Flammability
Inform patients and/or caregivers that YCANTH is flammable, even after drying. Instruct patients and/or caregivers to avoid fire, flame, or smoking near the lesions treated with YCANTH until YCANTH is removed [see Warnings and Precautions (5.3)].
Important Dosage and Administration Information
Inform patients and/or caregivers to remove YCANTH with soap and water 24 hours after treatment. Inform patients and/or caregivers that more than one YCANTH treatment may be necessary [see Dosage and Administration (2.2)].
-
SPL UNCLASSIFIED SECTION
Manufactured by:
Pharmaceutical Packaging Solutions
341 JD Yarnell
Industrial Pkwy,
Clinton, TN 37716For:
Verrica Pharmaceuticals Inc.
44 West Gay Street, Suite 400
West Chester, PA 19380YCANTH and VERRICA are registered trademarks of Verrica Pharmaceuticals Inc.
Copyright © 2024 Verrica Pharmaceuticals Inc.
All rights reserved. -
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 10/2024 PATIENT INFORMATION
YCANTH (WYE' KANTH)
(cantharidin)
topical solutionWhat is YCANTH?
YCANTH is a prescription medicine used on the skin (topical) to treat molluscum contagiosum (molluscum bumps) in adults and children 2 years of age and older.
It is not known if YCANTH is safe and effective in children under 2 years of age.Do not receive treatment with YCANTH if you are allergic to any of the ingredients in YCANTH. See the end of this leaflet for a complete list of ingredients in YCANTH. Before receiving treatment with YCANTH, tell your healthcare provider about all of your medical conditions, including if you: - are being treated or have had treatments for molluscum bumps.
- have other skin problems.
- are pregnant or plan to become pregnant. It is not known if YCANTH can harm your unborn baby.
- are breastfeeding or plan to breastfeed. Your healthcare provider should avoid applying YCANTH to areas of your skin where your baby could come into contact with YCANTH and get it in their mouth or eyes. Talk to your healthcare provider about whether you should breastfeed while YCANTH is on your skin.
How will I receive treatment with YCANTH?
- Your healthcare provider will apply YCANTH to the areas on your skin with molluscum bumps.
- YCANTH is for use on the skin (topical use) only. YCANTH is not for use in your mouth, nose, genital areas, or in your eyes.
- You may need more than 1 treatment with YCANTH. Your healthcare provider may apply YCANTH about every 3 weeks as needed.
- On the day of treatment, do not apply topical steroids, creams, lotions, or sunscreen to areas with molluscum bumps.
- Allow YCANTH to dry completely before leaving your healthcare provider's office or clinic (up to 5 minutes).
- Do not touch the treated areas for 24 hours or until washing.
- Do not allow treated areas of your skin to come into contact with your mouth, nose, genital areas, or your eyes for 24 hours or until washing.
- Do not allow children to lick or bite the treated areas. YCANTH contains a bittering agent to discourage young children from putting it in their mouths. See "What are the ingredients in YCANTH?"
- Do not apply topical steroids, creams, lotions, or sunscreen to treated areas for 24 hours after treatment or until you wash YCANTH off of your skin.
- Do not cover any of the treated areas with bandages.
- YCANTH contains a violet-colored dye. You may temporarily see the violet color on the treated areas of your skin.
- You may take over-the-counter pain relief medicines as needed.
- Wash the treated skin area with soap and water 24 hours after treatment unless your healthcare provider instructs you otherwise. You may wash YCANTH off of your skin sooner if you develop severe blistering, severe pain or other severe reactions. Do not use a washcloth, abrasive material or vigorous rubbing because this could be painful.
- Contact your healthcare provider with any questions.
What should I avoid after receiving treatment with YCANTH? - Avoid contact with the treated areas of your skin, including contact with your mouth (oral contact).
- Avoid applying other products to the treated areas of your skin until 24 hours after receiving treatment with YCANTH or until after washing YCANTH off of the treated areas of your skin.
- YCANTH is flammable, even after drying. Avoid fire, flame or smoking near lesion(s) during treatment and after treatment until YCANTH is washed off of your skin.
What are the possible side effects of YCANTH?
YCANTH can cause serious side effects, including:- Serious side effects if YCANTH is swallowed or it comes into contact with your mouth or eyes.
- YCANTH can cause life threatening reactions or death if it gets into your or your child's mouth or is swallowed. These reactions can include:
- kidney failure
- blisters and severe damage to your stomach and intestine (intestinal tract)
- blood clotting problems
- seizures
- a certain type of paralysis
If you or your child swallow (ingest) YCANTH, contact a Poison Control Center (1-800-222-1222) or go to the nearest hospital emergency room right away. - Eye contact reactions. Severe eye injury can happen if YCANTH gets into your eyes. Contact with YCANTH in the eyes may cause:
- ulcers or small holes in your eyes
- scarring
- redness
- irritation
- severe eye pain
- permanent eye injury, including blindness
If YCANTH accidentally gets into your eyes, flush them well with water for at least 15 minutes and get medical help right away. - Skin reactions. Skin reactions are common at the areas where you have been treated with YCANTH, but can also sometimes be severe. Skin reactions with YCANTH may include:
- small fluid-filled or non-fluid filled raised blisters on or around treated area(s)
- pain
- itching
- scabs
- redness
- lightening or darkening of the skin in the treated area(s)
- dry skin at the treated area(s)
- swelling on or near the treated area(s)
- breakdown of the outer layer of skin at the treated area(s)
If you develop severe blistering, severe pain or other severe reactions at the treated areas of your or your child's skin, wash YCANTH off of your skin sooner than 24 hours and call your healthcare provider. See "How will I receive treatment with YCANTH?" Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Verrica Pharmaceuticals Inc. at 1-877-837-7422.General information about the safe and effective use of YCANTH.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or healthcare provider for information that is written for health professionals.What are the ingredients in YCANTH?
Active ingredient: cantharidin
Inactive ingredients: acetone, camphor, castor oil, denatonium benzoate, ethanol, gentian violet, hydroxypropyl cellulose, and nitrocellulose. The inactive ingredient denatonium benzoate is a bittering agent to discourage young children from putting it in their mouths and accidentally swallowing YCANTH.
Manufactured by: Pharmaceutical Packaging Solutions, 341 JD Yarnell Industrial Pkwy, Clinton, TN 37716
For: Verrica Pharmaceuticals Inc., 44 West Gay Street, Suite 400, West Chester, PA 19380.
YCANTH and VERRICA are registered trademarks of Verrica Pharmaceuticals Inc.
Copyright © 2024 Verrica Pharmaceuticals Inc.
All rights reserved -
PRINCIPAL DISPLAY PANEL - 12 Ampule Applicator Package Carton
FOR ADMINISTRATION BY A HEALTHCARE PROFESSIONAL ONLY
NDC: 71349-070-12
Rx Only12 Applicators
and 2 Break ToolsYcanth®
(cantharidin)
TOPICAL
SOLUTION 0.7%Single use applicator contains
approximately 0.45 mL of 0.7% cantharidin solution.
Each 1 mL of solution contains 7 mg cantharidin.
Recommended Dosage: See prescribing information.
For Topical Use Only.- WARNING: Flammable liquid, even after drying. Avoid
fire, flame or smoking near lesion(s) during treatment
and after application until removed. - WARNING: Highly Toxic! For Topical Use Only.
YCANTH can be fatal if administered orally or taken
internally. - Wear gloves and eye protection during YCANTH®
preparation and administration. - Remove the paperboard sleeve and inspect the
applicator before using the break tool. - If YCANTH contacts healthy skin, immediately
remove by wiping with a cotton swab or gauze. - If YCANTH gets in the eyes, flush eyes with water
for at least 15 minutes and seek medical attention.
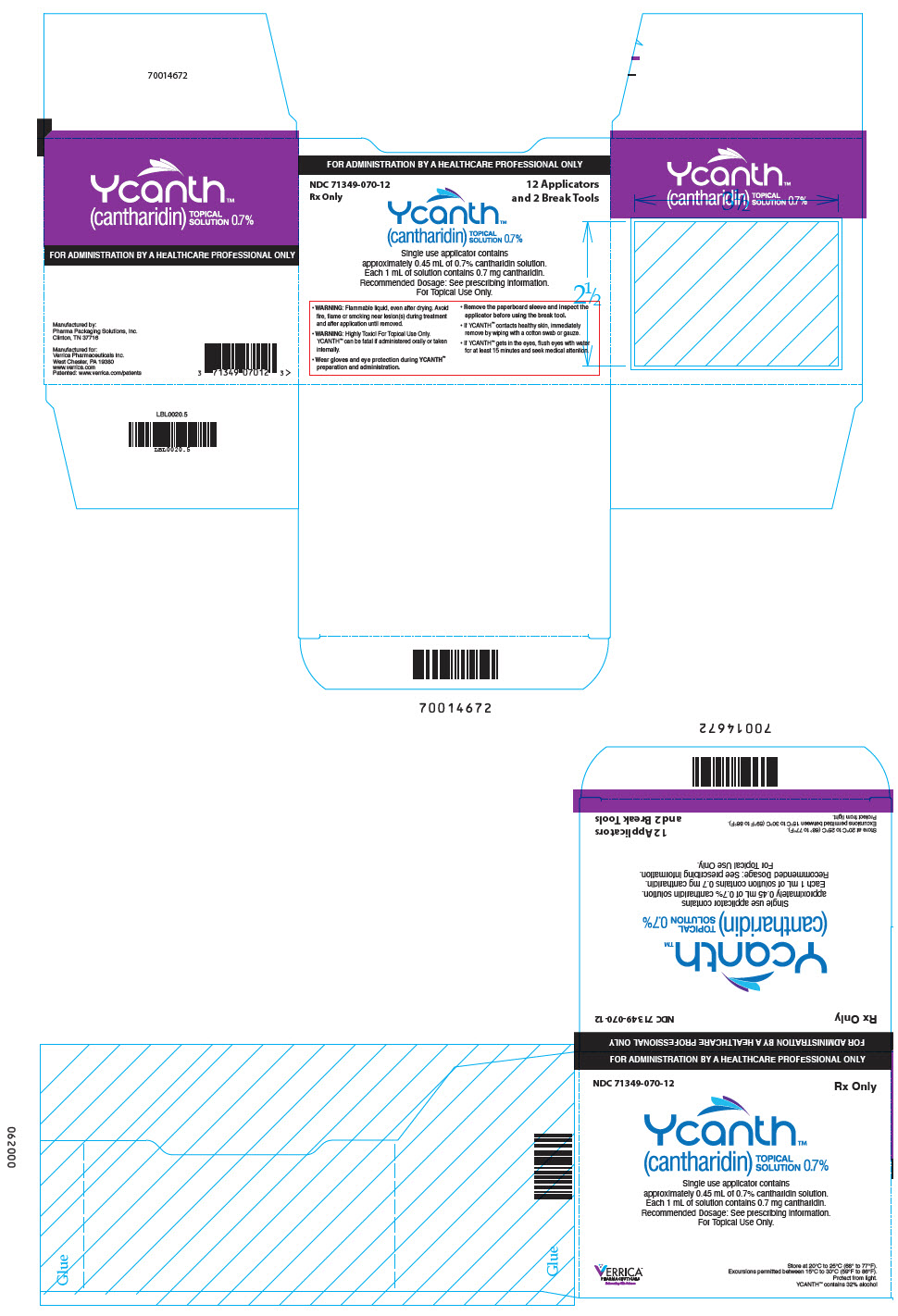
- WARNING: Flammable liquid, even after drying. Avoid
-
PRINCIPAL DISPLAY PANEL - 6 Ampule Applicator Package Carton
FOR ADMINISTRATION BY A HEALTHCARE PROFESSIONAL ONLY
NDC: 71349-999-06
Rx Only6 Applicators
and 2 Break ToolsYcanth®
(cantharidin)
TOPICAL
SOLUTION 0.7%Single use applicator contains
approximately 0.45 mL of 0.7% cantharidin solution.
Each 1 mL of solution contains 7 mg cantharidin.
Recommended Dosage: See prescribing information.
For Topical Use Only.Professional Sample. Not for Sale.
- WARNING: Flammable liquid, even after drying. Avoid
fire, flame or smoking near lesion(s) during treatment
and after application until removed. - WARNING: Highly Toxic! For Topical Use Only.
YCANTH can be fatal if administered orally or taken
internally. - Wear gloves and eye protection during YCANTH®
preparation and administration. - Remove the paperboard sleeve and inspect the
applicator before using the break tool. - If YCANTH contacts healthy skin, immediately
remove by wiping with a cotton swab or gauze. - If YCANTH gets in the eyes, flush eyes with water
for at least 15 minutes and seek medical attention.
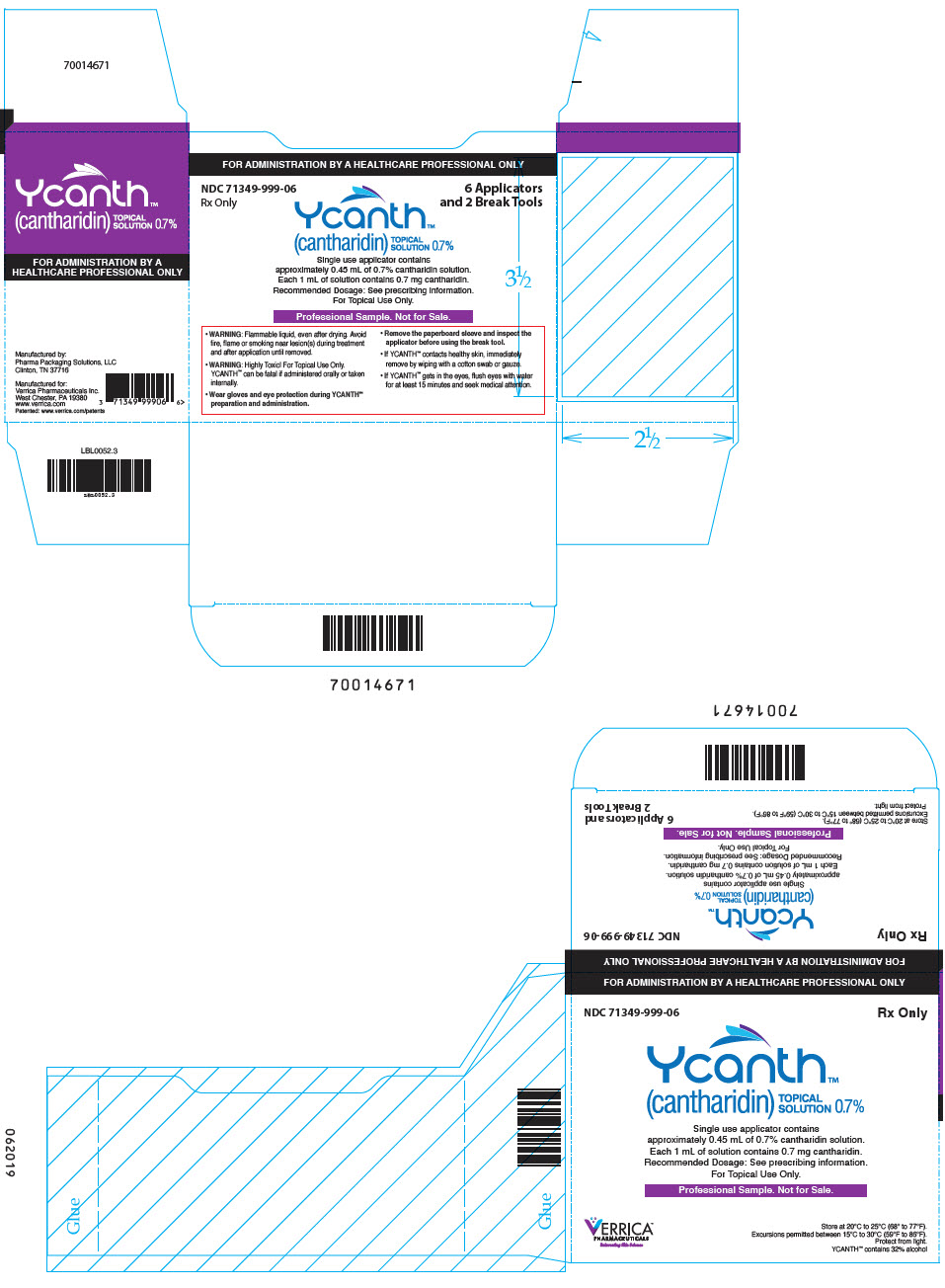
- WARNING: Flammable liquid, even after drying. Avoid
-
INGREDIENTS AND APPEARANCE
YCANTH
cantharidin solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71349-070 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Cantharidin (UNII: IGL471WQ8P) (cantharidin - UNII:IGL471WQ8P) Cantharidin 3.20 mg in 0.45 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71349-070-12 12 in 1 CARTON 07/21/2023 1 NDC: 71349-070-01 1 in 1 PACKAGE 1 1 in 1 APPLICATOR 1 0.45 mL in 1 AMPULE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC: 71349-070-11 1 in 1 CARTON 07/21/2023 2 NDC: 71349-070-01 1 in 1 PACKAGE 2 1 in 1 APPLICATOR 2 0.45 mL in 1 AMPULE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 3 NDC: 71349-070-06 6 in 1 CARTON 07/21/2023 3 NDC: 71349-070-01 1 in 1 PACKAGE 3 1 in 1 APPLICATOR 3 0.45 mL in 1 AMPULE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 4 NDC: 71349-070-01 1 in 1 PACKAGE 07/21/2023 4 1 in 1 APPLICATOR 4 0.45 mL in 1 AMPULE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212905 07/21/2023 YCANTH
cantharidin solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71349-999 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Cantharidin (UNII: IGL471WQ8P) (cantharidin - UNII:IGL471WQ8P) Cantharidin 3.20 mg in 0.45 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71349-999-06 6 in 1 CARTON 07/21/2023 1 NDC: 71349-999-01 1 in 1 PACKAGE 1 1 in 1 APPLICATOR 1 0.45 mL in 1 AMPULE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC: 71349-999-01 1 in 1 PACKAGE 07/21/2023 2 1 in 1 APPLICATOR 2 0.45 mL in 1 AMPULE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212905 07/21/2023 Labeler - Verrica Pharmaceuticals Inc. (015664551)
Trademark Results [YCANTH]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 YCANTH 97769376 not registered Live/Pending |
Verrica Pharmaceuticals Inc. 2023-01-26 |
 YCANTH 88862941 not registered Live/Pending |
Verrica Pharmaceuticals Inc. 2020-04-07 |
 YCANTH 88862892 not registered Live/Pending |
Verrica Pharmaceuticals Inc. 2020-04-07 |
 YCANTH 88550445 not registered Live/Pending |
Verrica Pharmaceuticals Inc. 2019-07-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.