HYQVIA (immune globulin 10 percent- human with recombinant human hyaluronidase kit
HYQVIA by
Drug Labeling and Warnings
HYQVIA by is a Other medication manufactured, distributed, or labeled by Baxalta US Inc., Baxalta Belgium Manufacturing SA, Baxter Pharmaceutical Solutions, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use HYQVIA safely and effectively. See full prescribing information for HYQVIA.
HYQVIA [Immune Globulin Infusion 10% (Human) with Recombinant Human Hyaluronidase]
Solution for subcutaneous administration
Initial U.S. Approval: 2014WARNING: THROMBOSIS
See full prescribing information for complete boxed warning
- Thrombosis may occur with immune globulin products, including HYQVIA. Risk factors may include advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling vascular catheters, hyperviscosity and cardiovascular risk factors.
- For patients at risk of thrombosis, administer HYQVIA at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration.
- Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk of hyperviscosity.
RECENT MAJOR CHANGES
Dosage and Administration (2.4) 2/2020 INDICATIONS AND USAGE
HYQVIA is an immune globulin with a Recombinant Human Hyaluronidase indicated for the treatment of Primary Immunodeficiency (PI) in adults. (1)
Limitation of Use:
Safety and efficacy of chronic use of Recombinant Human Hyaluronidase in HYQVIA have not been established in conditions other than PI.
DOSAGE AND ADMINISTRATION
For subcutaneous use only.
Initial Treatment Interval/Dosage Ramp-Up Schedule
Week
Infusion Number
Dose Interval
Example for 30 grams per 4 weeks
1
1st infusion
1-week-dose
7.5 grams
2
2nd infusion
2-week-dose
15 grams
3
No infusion
4
3rd infusion
3-week-dose
22.5 grams
5
No infusion
6
No infusion
7
4th infusion (if required)
4-week-dose
30 grams
Naïve to Immune Globulin Subcutaneous (Human) [IGSC] treatment or switching from IGSC: 300 to 600 mg/kg at 3 to 4 week intervals, after initial ramp-up. (2.1)
Switching from Immune Globulin Intravenous (Human) [IGIV] treatment: Use same dose and frequency as previous intravenous treatment after the initial ramp-up. (2.1)
DOSAGE FORMS AND STRENGTHS
- ▪ A dual vial unit containing 10% IgG (100 mg/mL) and 160 U/mL Recombinant Human Hyaluronidase (3). HYQVIA is available in the following strengths:
Immune Globulin Infusion 10% (Human)
Recombinant Human Hyaluronidase
Grams Protein
Units
2.5
200
5.0
400
10.0
800
20.0
1600
30.0
2400
CONTRAINDICATIONS
- History of anaphylactic or severe systemic hypersensitivity reactions to Immune Globulin (Human). (4)
- IgA deficient patients with antibodies against IgA and a history of hypersensitivity. (4)
- Known systemic hypersensitivity to hyaluronidase including Recombinant Human Hyaluronidase of HYQVIA.
- Known systemic hypersensitivity to human albumin (in the hyaluronidase solution). (4)
WARNINGS AND PRECAUTIONS
- IgA-deficient patients with anti-IgA antibodies are at greater risk of severe hypersensitivity and anaphylactic reactions. (5.1)
- Thrombosis may occur following treatment with immune globulin products including HYQVIA. (5.2)
- Antibodies to PH20 (Recombinant Human Hyaluronidase) can develop. The potential exists for such antibodies to cross-react with endogenous PH20 which is known to be expressed in the adult male testes, epididymis, and sperm. It is unknown whether these antibodies may interfere with fertilization in humans. The clinical significance of these antibodies is not known. (5.3)
- Aseptic Meningitis Syndrome (AMS) may occur. Discontinue treatment if AMS symptoms appear. (5.4)
- Acute intravascular hemolysis may occur. Monitor for clinical signs and symptoms of hemolysis and hemolytic anemia. (5.5)
- Infusion into or around an infected area can spread a localized infection. (5.7)
- Monitor for pulmonary adverse reactions (transfusion-related acute lung injury [TRALI]). (5.8)
- May carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. (5.9)
ADVERSE REACTIONS
The most common adverse reactions observed in clinical trials in >5% of subjects were: local reactions, headache, antibody formation against Recombinant Human Hyaluronidase (rHuPH20), fatigue, nausea, pyrexia, and vomiting. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Baxalta US Inc., a Takeda company at 1-800-999-1785 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Passive transfer of antibodies may transiently interfere with the immune responses to live virus vaccines, such as measles, mumps, and rubella. (7)
USE IN SPECIFIC POPULATIONS
- Geriatric: In patients over age 65 or in any patient at risk of developing renal insufficiency, do not exceed the recommended dose, and consider infusing HYQVIA at lower, more frequent doses. (8.5)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: THROMBOSIS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Administration
2.3 Preparation and Handling
2.4 Instructions for Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
5.2 Thrombosis
5.3 Immunogenicity of Recombinant Human Hyaluronidase (PH20)
5.4 Aseptic Meningitis Syndrome (AMS)
5.5 Hemolysis
5.6 Renal Dysfunction/Failure
5.7 Spread of Localized Infection
5.8 Transfusion-Related Acute Lung Injury (TRALI)
5.9 Transmittable Infectious Agents
5.10 Interference with Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: THROMBOSIS
- Thrombosis may occur with immune globulin products, including HYQVIA. Risk factors may include advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling vascular catheters, hyperviscosity and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors.
- For patients at risk of thrombosis, administer HYQVIA at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration.
- Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk of hyperviscosity.
-
1 INDICATIONS AND USAGE
HYQVIA is an immune globulin with a Recombinant Human Hyaluronidase indicated for the treatment of Primary Immunodeficiency (PI) in adults. This includes, but is not limited to, common variable immunodeficiency (CVID), X-linked agammaglobulinemia, congenital agammaglobulinemia, Wiskott-Aldrich syndrome, and severe combined immunodeficiencies1,2.
Limitation of Use:
Safety and efficacy of chronic use of Recombinant Human Hyaluronidase in HYQVIA have not been established in conditions other than PI.
-
2 DOSAGE AND ADMINISTRATION
For subcutaneous use only.
2.1 Dosage
Initiation of Treatment with HYQVIA
- For patients previously on another IgG treatment, administer the first dose approximately one week after the last infusion of their previous treatment.
- Increase the dose and frequency from a 1-week dose to a 3- or 4-week dose (see ramp-up schedule in Table 1).
- Initiating treatment at a full monthly dose was not evaluated in the clinical trial.
Table 1 Initial Treatment Interval/Dosage Ramp-Up Schedule Week
Infusion Number
Dose Interval
Example for 30 grams per 4 weeks
1
1st infusion
1-week-dose
7.5 grams
2
2nd infusion
2-week-dose
15 grams
3
No infusion
4
3rd infusion
3-week-dose
22.5 grams
5
No infusion
6
No infusion
7
4th infusion (if required)
4-week-dose
30 grams
For patients switching from Immune Globulin Intravenous (Human) [IGIV] treatment:
Administer HYQVIA at the same dose and frequency as the previous intravenous treatment, after the initial dose ramp-up.
For patients naïve to Immune Globulin Subcutaneous (Human) [IGSC] treatment or switching from IGSC:
Administer HYQVIA at 300 to 600 mg/kg at 3 to 4 week intervals, after initial ramp-up.
Individualization of Dose
If HYQVIA is administered at the same dose and frequency, the serum IgG levels from HYQVIA should be comparable to serum IgG levels from intravenous treatment.
For dose adjustment:
- Calculate the difference between the patient's serum IgG trough level during HYQVIA treatment and the IgG trough level during the previous intravenous treatment.
- Find this difference (in mg/dL) in the columns of Table 2 and the corresponding amount (in mL) by which to increase or decrease the dose based on the patient's body weight and desired change in IgG trough level.
Table 2 Individualization in Volume Administered per Dosing Interval for Intended Change in IgG Trough Level* Difference in IgG Trough Levels Body Weight 100 mg/dL 200 mg/dL 300 mg/dL 400 mg/dL - * Derived using a slope of 3.3 kg/dL
10 kg
3 mL
6 mL
9 mL
12 mL
20 kg
6 mL
12 mL
18 mL
24 mL
30 kg
9 mL
18 mL
27 mL
36 mL
40 kg
12 mL
24 mL
36 mL
48 mL
50 kg
15 mL
30 mL
45 mL
61 mL
60 kg
18 mL
36 mL
55 mL
73 mL
70 kg
21 mL
42 mL
64 mL
85 mL
80 kg
24 mL
48 mL
73 mL
97 mL
90 kg
27 mL
55 mL
82 mL
109 mL
100 kg
30 mL
61 mL
91 mL
121 mL
110 kg
33 mL
67 mL
100 mL
133 mL
120 kg
36 mL
73 mL
109 mL
145 mL
130 kg
39 mL
79 mL
118 mL
158 mL
140 kg
42 mL
85 mL
127 mL
170 mL
Example 1: A patient with a body weight of 80 kg has a measured IgG trough level of 800 mg/dL and the reference trough level is 1000 mg/dL. The trough level difference is 200 mg/dL (1000 mg/dL minus 800 mg/dL). The dose of HYQVIA would be increased by 48 mL (4.8 grams) per dosing interval.
Example 2: A patient with a body weight of 60 kg has a measured IgG trough level of 1000 mg/dL and the reference trough level is 900 mg/dL. The trough level difference is -100 mg/dL (900 mg/dL minus 1000 mg/dL). The dose of HYQVIA would be decreased by 18 mL (1.8 grams) per dosing interval.
HYQVIA can be used to administer a full therapeutic dose in one site up to every four weeks. Adjust the frequency and number of infusion sites taking into consideration volume, total infusion time, and tolerability. Adjust the frequency as needed so that the patient receives the same weekly equivalent dose.
Example: When adjusting a dose of 30 grams administered every 3 weeks, administer 40 grams of HYQVIA every 4 weeks. If a higher trough level is required relative to intravenous treatment at 3 or 4 week intervals, increase the dose or decrease the dosing interval. Evaluate the use of a second site or infusing at shorter intervals when the volume of HYQVIA is greater than 600 mL.
If a patient misses a dose, administer the missed dose as soon as possible and then resume scheduled treatments as applicable.
If HYQVIA is administered at a different interval than the previous treatment, either intravenously or subcutaneously, then Table 2 should not be used and the dose of HYQVIA should be adjusted, if necessary, based on clinical response.
2.2 Administration
HYQVIA should be administered by a healthcare professional, caregiver or self-administered by the patient after appropriate training.
-
Infusion of Immune Globulin Infusion 10% (Human) requires an infusion pump capable of infusing a patient's therapeutic dose at infusion rates up to 300 mL/hr/site. The pump must have the ability to titrate the flow rate up or down if required to improve tolerability. To ensure maximum flow rates, use a subcutaneous needle set that is 24 gauge and labeled for high flow rates.
- Infusion site leakage can occur during or after subcutaneous administration of immunoglobulin, including HYQVIA. Consider using longer needles (14 or 12 mm rather than 9 mm) and/or more than one infusion site.
- Infuse the two components of HYQVIA sequentially, beginning with the Recombinant Human Hyaluronidase.
- Initiate infusion of the full dose of the Immune Globulin Infusion 10% (Human) through the same subcutaneous needle set within approximately 10 minutes of the Recombinant Human Hyaluronidase infusion.For each full or partial vial of Immune Globulin Infusion 10% (Human) used, administer the entire contents of the Recombinant Human Hyaluronidase vial.
Selection of Infusion Site(s)
The suggested site(s) for the infusion of HYQVIA are the abdomen and thighs. If two sites are used, the two infusion sites should be on opposite sides of the body. Avoid bony prominences, or areas that are scarred, inflamed or infected.
Volume per Site
Administer up to 600 mL per site for patients whose body weight is greater than or equal to 40 kg and up to 300 mL per site for patients whose body weight is less than 40 kg.
A second site can be used at the discretion of the physician and patient based on tolerability and total volume. If a second site is used, administer half the total volume of Recombinant Human Hyaluronidase of HYQVIA in each site.
Rate of Infusion
Administer the Recombinant Human Hyaluronidase of HYQVIA at an initial rate per site of approximately 1 to 2 mL per minute, or as tolerated.
Administer Immune Globulin Infusion 10% (Human) of HYQVIA at rates as shown in Table 3 for the initial infusions. If the patient tolerates these infusions at the full dose and maximum rate, adjust both the time intervals and number of rate changes of the ramp-up used for successive infusions at the discretion of the physician and patient.
Table 3 Immune Globulin Infusion 10% (Human) Infusion Rates First 2 Infusions
Subsequent 2 or 3 Infusions
Subjects < 40 kg (< 88lbs)
Subjects ≥ 40 kg (≥ 88lbs)
Subjects < 40 kg (< 88lbs)
Subjects ≥ 40 kg (≥ 88lbs)
Intervals
Rate per site
Rate per site
Rate per site
Rate per site
Minutes
mL per hour
mL per hour
mL per hour
mL per hour
- 5 - 15
5
10
10
10
- 5 - 15
10
30
20
30
- 5 - 15
20
60
40
120
- 5 - 15
40
120
80
240
Remainder of infusion
80
240
160
300
2.3 Preparation and Handling
-
Visually inspect both vials of HYQVIA for discoloration and particulate matter prior to administration.
- o The appearance of the Immune Globulin Infusion 10% (Human) of HYQVIA can vary from clear or slightly opalescent and colorless or pale yellow.
- o The appearance of the Recombinant Human Hyaluronidase of HYQVIA should be clear and colorless.
- o Do not use either component of HYQVIA if either solution is cloudy or has particulates.
- Allow refrigerated product to come to room temperature before use. Do not apply heat or place in microwave.
- Do not shake HYQVIA.
- Do not mix the Recombinant Human Hyaluronidase and the Immune Globulin Infusion 10% (Human) of HYQVIA into the same container prior to administration.
- Do not mix or administer components of HYQVIA with other products. Administer components of HYQVIA sequentially. Do not use either component alone.
- Flush the infusion line with normal saline or Dextrose 5% in water (D5W) if required.
- HYQVIA contains no preservative. Discard any unused product according to local standards for biohazard products.
2.4 Instructions for Administration
Use aseptic technique when preparing and administering HYQVIA for infusion.
For more detail on steps, see accompanying Information for Patients.
- 1. Inspect the vials: Inspect for clarity, color, and expiration date(s)
- 2. Prepare for infusion:
- Gather supplies: HYQVIA dual vial unit(s), ancillary supplies, sharps container and infusion pump (program pump per physician recommendation following manufacturer's instructions).
- Prepare a clean work area.
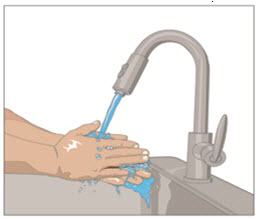
- Wash hands.
- If the Immune Globulin Infusion 10% (Human) and Recombinant Human Hyaluronidase are pooled into separate containers, skip to Step 5.
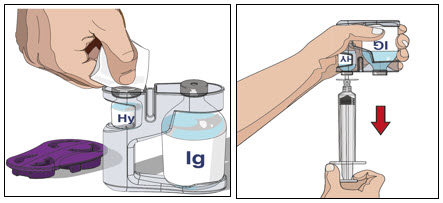
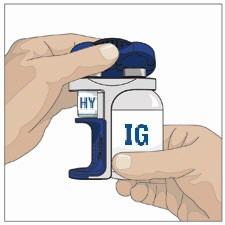
- 3. Prepare the Recombinant Human Hyaluronidase of HYQVIA (Labeled as "HY"):
- Remove the protective cap.
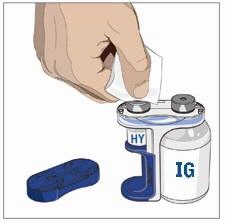
- Wipe each stopper with a sterile alcohol wipe and allow to dry.
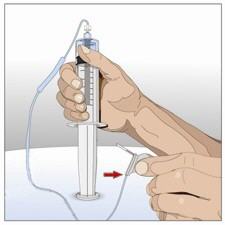
- Attach a syringe to a needle/needle-less transfer device. A sterile needle or needle-less transfer device (18-22 gauge sterile needle) may be used for all vial sizes. Position the sharp tip of the needle/needle-less transfer device over the center of the vial stopper and insert it at a 90-degree angle. Inject air and then draw the full contents of each vial labeled "HY" into a single syringe, if possible.
- Attach the syringe containing the Recombinant Human Hyaluronidase to the subcutaneous needle set and prime up to the needle hub.
- 4. Prepare Immune Globulin Infusion 10% (Human) of HYQVIA (Labeled as "IG"):
- Wipe each stopper with a sterile alcohol wipe and allow to dry.
-
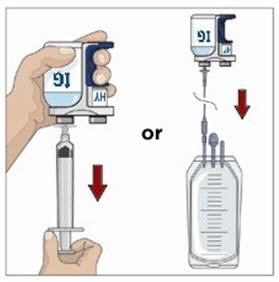
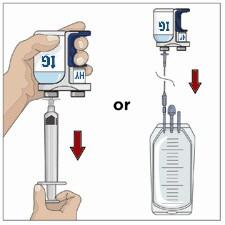
Transfer the vial(s) labeled "IG" using one of the following methods:
- o Pool into an infusion bag, using a transfer device per manufacturer's directions. Attach and prime the pump administrating tubing; or
- o Directly spike the vial using a vented pump administration tubing and prime as directed.
If using a syringe driver, transfer into the syringe(s), preferably using a vented spike.
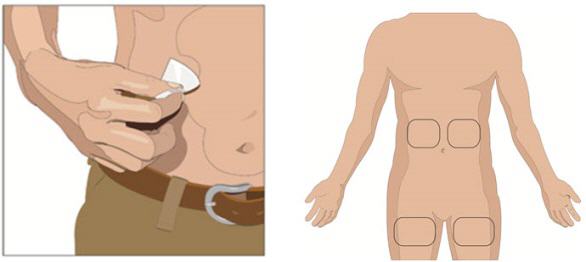
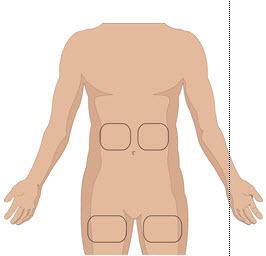
- 5. Prepare the infusion site(s):
- Potential sites for infusion include the middle to upper abdomen and thigh.
- Avoid: bony prominences, blood vessels, scars, or areas that are inflamed or infected.
- If two sites are desired, a bifurcated needle set may be used on opposite sides of the body.
- Rotate sites by choosing opposite sides of the body between successive infusions.
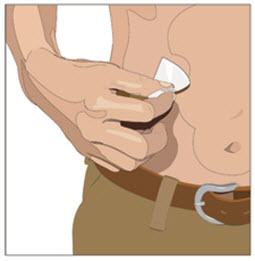
- Cleanse the infusion site(s) with a sterile alcohol wipe beginning at the center of each infusion site and moving outward in a circular motion. Allow the infusion site(s) to dry.
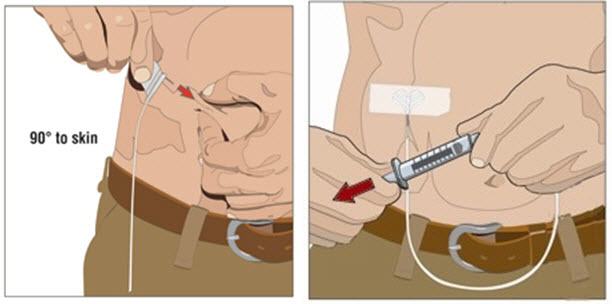
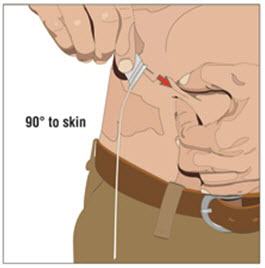
- 6. Insert and secure the 24 gauge subcutaneous needle:
- Pinch at least one inch of skin between two fingers. Insert the needle at a 90-degree angle into the subcutaneous tissue and secure the needle with sterile tape.
-
Check placement: gently pull back on the plunger of the attached syringe and monitor for any blood return in the tubing.
- o If blood is seen in the tubing, remove and discard the needle and repeat steps 3, 5 and 6 with a new subcutaneous needle and infusion site.
- Secure the needle in place with a sterile protective dressing.
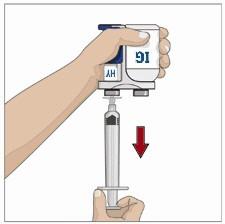
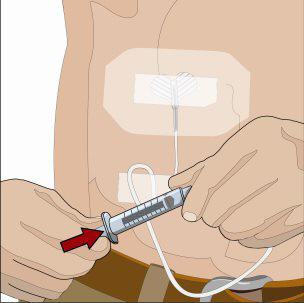
- 7. Administer the Recombinant Human Hyaluronidase of HYQVIA:
Infuse the Recombinant Human Hyaluronidase manually at an initial rate of approximately 1 to 2 mL per minute per infusion site and increase as tolerated. If more than one site is used, divide the contents equally between sites. At the end of infusion, disconnect the empty syringe and attach the pump tubing/syringe containing the Immune Globulin Infusion 10% (Human) of HYQVIA to the same subcutaneous needle set.
- 8. Administer the Immune Globulin Infusion 10% (Human) of HYQVIA:
Within approximately 10 minutes of completing the infusion of the Recombinant Human Hyaluronidase of HYQVIA, start the variable rate program of the infusion pump to initiate the infusion of the full therapeutic dose of Immune Globulin Infusion 10% (Human) of HYQVIA. At the end of infusion, flush the infusion tubing up to the needle with normal saline or Dextrose 5% in water (D5W), if required.
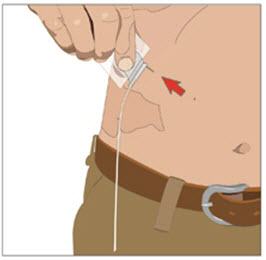
- 9. Remove subcutaneous needle(s) from the infusion site(s):
After the infusion is complete, remove the needle set and cover with a protective dressing. Discard any partially used vial(s) and disposable supplies in accordance with local requirements.
- 10. Document the infusion:
Remove the peel-off label from each Immune Globulin Infusion 10% (Human) vial of HYQVIA used and affix to the patient's treatment record or infusion log. In addition, record the time, date, dose, infusion site location and any reactions after each infusion.
For self-administration, provide the patient with instructions and training for infusion in the home or other appropriate setting.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
HYQVIA is contraindicated in:
- Patients who have had a history of anaphylactic or severe systemic reactions to the administration of IgG.
- IgA deficient patients with antibodies to IgA and a history of hypersensitivity.
- Patients with known systemic hypersensitivity to hyaluronidase including Recombinant Human Hyaluronidase of HYQVIA.
Patients with known systemic hypersensitivity to human albumin (in the hyaluronidase solution)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
Severe hypersensitivity reactions may occur, even in patients who have tolerated previous treatment with IgG. In case of hypersensitivity, discontinue the HYQVIA infusion immediately and institute appropriate treatment.
Immune Globulin Infusion 10% (Human) of HYQVIA contains trace amount of IgA (average concentration of 37μg/mL). Patients with antibodies to IgA potentially are at greater risk of developing potentially severe hypersensitivity and anaphylactic reactions.
5.2 Thrombosis
Thrombosis may occur following treatment with immune globulin products, including HYQVIA. Risk factors may include advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling central vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors.
Consider baseline assessment of blood viscosity in patients at risk for hyperviscosity, such as those with cryoglobulins, fasting chylomicronemia/markedly high triacylglycerols (triglycerides), or monoclonal gammopathies. For patients at risk of thrombosis, administer HYQVIA at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity. [see Boxed Warning, Dosage and Administration (2), Patient Counseling Information (17)].
5.3 Immunogenicity of Recombinant Human Hyaluronidase (PH20)
Eighteen percent (15 of 83) of subjects receiving HYQVIA in clinical studies developed non-neutralizing antibodies to the Recombinant Human Hyaluronidase component. The potential exists for such antibodies to cross-react with endogenous PH20, which is known to be expressed in the adult male testes, epididymis, and sperm. It is unknown whether these antibodies can interfere with fertilization in humans. The clinical significance of these antibodies is not known.
5.4 Aseptic Meningitis Syndrome (AMS)
AMS has been reported to occur with IgG products, including Immune Globulin Infusion 10% (Human) administered intravenously and subcutaneously. AMS may occur more frequently in female patients. Discontinuation of IgG treatment has resulted in remission of AMS within several days without sequelae. The syndrome usually begins within several hours to two days following intravenously administered IgG.
AMS is characterized by the following signs and symptoms: severe headache, nuchal rigidity, drowsiness, fever, photophobia, painful eye movements, nausea and vomiting [see Patient Counseling Information (17)]. Cerebrospinal fluid (CSF) studies frequently reveal pleocytosis up to several thousand cells per mm3, predominantly from the granulocytic series, and elevated protein levels up to several hundred mg/dL, but negative culture results. Conduct a thorough neurological examination on patients exhibiting such symptoms and signs, including CSF studies, to rule out other causes of meningitis.
5.5 Hemolysis
IgG products, including HYQVIA, contain blood group antibodies which may act as hemolysins and induce in vivo coating of red blood cells (RBC) with IgG. These antibodies may cause a positive direct antiglobulin reaction and hemolysis6.Acute intravascular hemolysis has been reported following intravenously administered IgG, including Immune Globulin Infusion 10% (Human) administered intravenously, and delayed hemolytic anemia can develop due to enhanced RBC sequestration [see Adverse Reactions (6)].
Monitor patients for clinical signs and symptoms of hemolysis. If signs and/or symptoms of hemolysis are present after HYQVIA infusion, perform appropriate confirmatory laboratory testing [see Patient Counseling Information (17)].
5.6 Renal Dysfunction/Failure
Acute renal dysfunction/failure, acute tubular necrosis, proximal tubular nephropathy, osmotic nephrosis and death may occur upon use of IgG products administered intravenously, especially those containing sucrose4. HYQVIA does not contain sucrose. Acute renal dysfunction/failure has been reported in association with Immune Globulin Infusion 10% (Human) administered intravenously. Ensure that patients are not volume depleted prior to the initiation of infusion of HYQVIA. In patients who are at risk of developing renal dysfunction because of pre-existing renal insufficiency or predisposition to acute renal failure (such as diabetes mellitus, age greater than 65, volume depletion, sepsis, paraproteinemia, or patients receiving known nephrotoxic drugs), monitor renal function and consider lower, more frequent dosing.
Periodic monitoring of renal function and urine output is particularly important in patients judged to be at increased risk for developing acute renal failure. Assess renal function, including measurement of blood urea nitrogen (BUN) and serum creatinine, before the initial infusion of HYQVIA and again at appropriate intervals thereafter. If renal function deteriorates, consider discontinuation of HYQVIA.
5.7 Spread of Localized Infection
Infusion into or around an infected area can spread a localized infection. Do not infuse HYQVIA into these areas due to potential risk of spreading a localized infection.
5.8 Transfusion-Related Acute Lung Injury (TRALI)
Non-cardiogenic pulmonary edema (TRALI) may occur with intravenously administered IgG and has been reported to occur with Immune Globulin Infusion 10% (Human) administered intravenously. TRALI is characterized by severe respiratory distress, pulmonary edema, hypoxemia, normal left ventricular function, and fever. Symptoms typically occur within 1 to 6 hours after treatment.
Monitor patients for pulmonary adverse reactions [see Patient Counseling Information (17)]. If TRALI is suspected, conduct an evaluation, including appropriate tests for the presence of anti-neutrophil and anti-HLA antibodies in both the product and patient serum. TRALI may be managed using oxygen therapy with adequate ventilatory support.
5.9 Transmittable Infectious Agents
Because Immune Globulin Infusion 10% (Human) of HYQVIA is made from human plasma, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant CJD (vCJD) agent, and theoretically, the classic Creutzfeldt-Jakob disease agent. This also applies to unknown or emerging viruses and other pathogens. No cases of transmission of viral diseases or vCJD have been associated with HYQVIA.
Report all infections thought to be possibly transmitted by HYQVIA to Baxalta US Inc., at 1-800-423-2090 (in the U.S.).
5.10 Interference with Laboratory Tests
- After infusion of IgG, the transitory rise of the various passively transferred antibodies in the patient's blood may yield false positive serological testing results, with the potential for misleading interpretation. Passive transmission of antibodies to erythrocyte antigens (e.g., A, B, and D) may cause a positive direct or indirect antiglobulin (Coombs') test.
- Infusions of immune globulin products can lead to false positive readings in assays that depend on detection of ß-D-glucans for diagnosis of fungal infections; this may persist during the weeks following infusion of the product.
-
6 ADVERSE REACTIONS
Common adverse reactions observed in clinical trials in >5% of subjects were: local reactions, headache, antibody formation against Recombinant Human Hyaluronidase (rHuPH20), fatigue, nausea, pyrexia, and vomiting.
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in clinical studies of a product cannot be directly compared to rates in the clinical studies of another product and may not reflect the rates observed in clinical practice.
Immune Globulin Infusion 10% (Human) administered intravenously: Prior to initiation of treatment with HYQVIA, 87 patients received 365 infusions of immune globulin infusion 10% (Human) encompassing 22.2 patient-years. Among the 87 patients treated, 56 (64.4%) experienced 1 or more adverse reactions. Among the 365 intravenous infusions, 158 adverse reactions occurred for a rate per infusion of 0.43.
A total of 1359 infusions of HYQVIA were administered during the trial; 230 of these infusions occurred during the ramp-up period and the other 1129 occurred during the observation period. During the observation period, 81 patients received 1129 infusions of HYQVIA; of those, 67 (82.7%) experienced one or more adverse reactions. Among the 1129 HYQVIA infusions, 456 adverse reactions occurred for a rate per infusion of 0.40. Seven of these adverse reactions were severe, defined as marked impairment of function, can lead to temporary inability to resume normal life pattern, requires prolonged intervention and/or results in sequelae.
Adverse reactions occurring in greater than 5% of subjects associated with infusions of HYQVIA vs. Immune Globulin Infusion 10% (Human) given intravenously are shown in Table 4. The majority of these adverse reactions was mild to moderate in severity and did not necessitate discontinuing the infusions. Mild is defined as transient discomfort that resolves spontaneously or with minimal intervention; moderate is defined as limited impairment of function that resolves spontaneously or with minimal intervention with no sequelae. No serious adverse reactions occurred during the HYQVIA clinical trials.
Table 4 Adverse Reactions* in greater than 5% of Subjects Associated with Infusions of HYQVIA vs. Immune Globulin Infusion 10% (Human) (IGIV) Given Intravenously HYQVIA IGIV Given Intravenously Adverse Reactions† Number of Subjects (%)
N= 81Number of Adverse Reactions per Infusion (Rate‡)
N = 1129Number of Subjects (%)
N=87Number of Adverse Reactions per Infusion (Rate)
N = 365- * Causally related adverse events and/or temporally associated adverse events occurring within 72 hours.
- † Excluding infections
- ‡ Rate = total number of events divided by total number of infusions.
Local ARs
42 (51.9%)
234 (0.21)
4 (4.6%)
4 (0.01)
Systemic ARs
55 (67.9%)
222 (0.20)
54 (62.1%)
154 (0.42)
Headache
17 (21%)
40 (0.04)
22 (25.3%)
42 (0.12)
Fatigue
9 (11.1%)
16 (0.01)
8 (9.2%)
10 (0.03)
Nausea
6 (7.4%)
12 (0.01)
10 (11.5%)
10 (0.03)
Pyrexia
6 (7.4%)
11 (0.01)
6 (6.9%)
7 (0.02)
Vomiting
6 (7.4%)
11 (0.01)
5 (5.7%)
7 (0.02)
Six subjects, 2 children and 4 adults, withdrew from the trial during the efficacy treatment period with HYQVIA due to mild to moderate adverse reactions. One child withdrew due to local pain and one due to fever, vomiting, and headaches. Of the four adults, two withdrew due to local pain and swelling, one had moderate swelling that transiently extended from the abdominal infusion site to the genitalia, and one had back injury.
Antibodies binding to rHuPH20: A total of 15 out of 83 subjects in the clinical trial who were treated with HYQVIA developed an antibody capable of binding to Recombinant Human Hyaluronidase. These antibodies were not capable of neutralizing Recombinant Human Hyaluronidase.
In the clinical trial, no temporal association between adverse reactions and the presence of antibodies capable of binding to the Recombinant Human Hyaluronidase of HYQVIA could be demonstrated. There was no increase in the incidence or severity of adverse reactions in subjects who developed antibodies to Recombinant Human Hyaluronidase of HYQVIA. In all subjects, antibody titers decreased despite continued treatment.
The effect of exposure to antibodies capable of binding to Recombinant Human Hyaluronidase of HYQVIA for periods longer than this clinical trial has not been evaluated.
The local adverse reactions are listed by frequency in Table 5. Mild swelling around the infusion site was present in most infusions due to the large volumes infused, but in general was not considered to be an adverse reaction unless it caused discomfort. Among the 234 local adverse reactions, three were severe (infusion site pain, infusion site swelling and infusion site edema that extended from the abdominal infusion site to the genitalia); all were transient and resolved without sequelae. More than 98% of local reactions were either mild (70.5%) or moderate (28.2%) in severity.
Table 5 Most Frequent Local Adverse Reactions Reported in greater than 1% of Infusion During Treatment With HYQVIA Infusion Site Reaction Number and Rate of Reactions per Infusion
N=1129Rate per infusion = total number of events divided by total number of infusions Discomfort/pain
122 (0.11)
Erythema
32 (0.03)
Swelling/Edema
35 (0.03)
Pruritus
22 (0.02)
During the combined efficacy and extension trials encompassing more than 3 years, the local adverse reaction rate was 2.6 per patient-year. During the first 12 month period (months 1-12), the rate was 3.68 local adverse reactions per patient-year. During the subsequent 12 month period (months 13-24), the rate declined to 2.12 local adverse reactions per-patient year. Finally, during the third 12 month period (months 25-36), the rate further declined to 0.37 local adverse reactions per patient-year.
Sixty-six of the 68 subjects who completed the efficacy clinical trial enrolled in a prospective, open-label, multicenter extension trial to assess the long-term safety and tolerability of HYQVIA. Sixty-three of 66 subjects enrolled received HYQVIA and 3 received IGIV. Of the 63 subjects who received HYQVIA, 48 completed the extension trial. The cumulative exposure of HYQVIA across the two trials was 188 subject-years and 2959 infusions, and a maximum exposure of 188 weeks or up to approximately 3.5 years. There were no clinically observable changes in the skin or subcutaneous tissue in either the efficacy or extension clinical trials.
6.2 Postmarketing Experience
Because postmarketing reporting of adverse reactions is voluntary and from a population of uncertain size, it is not always possible to reliably estimate the frequency of these reactions or establish a causal relationship to product exposure.
In addition to the adverse reactions listed above in clinical trials, the following adverse reactions have been reported in the postmarketing experience:
Immune System Disorder: Hypersensitivity
General Disorder and Administration Site Conditions: Influenza-like illness, Infusion site leaking
Postmarketing Experience of Immune Globulin Products
The following adverse reactions have been identified and reported during the postmarketing use of Immune Globulin products administered subcutaneously:
Anaphylactic reaction, Tremor, Tachycardia, Hypotension, Infusion related reaction, Dyspnea, Paresthesia oral, Dermatitis allergic, Injection site rash, and Alanine aminotransferase increased.
-
7 DRUG INTERACTIONS
Passive transfer of antibodies can transiently impair the immune responses to live attenuated virus vaccines, such as mumps, rubella and varicella for up to 6 months and for a year or more to measles. [see Patient Counseling Information (17)].
Admixtures of HYQVIA with other drugs solutions have not been evaluated. Do not mix or administer components of HYQVIA with other products.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
No human data are available to indicate the presence or absence of drug-associated risk. Animal reproduction studies have not been conducted with Immune Globulin Infusion 10% (Human) component of HYQVIA. It is not known whether HYQVIA can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Immune globulins cross the placenta from maternal circulation increasingly after 30 weeks of gestation.
Development and reproductive toxicology studies have been conducted with Recombinant Human Hyaluronidase in mice and rabbits [see Animal Toxicology and/or Pharmacology (13.2)]. No adverse effects on pregnancy were associated with anti-rHuPH20 antibodies. In these studies, maternal antibodies to Recombinant Human Hyaluronidase were transferred to offspring in utero. The effects of antibodies to the Recombinant Human Hyaluronidase component of HYQVIA on the human embryo or on human fetal development are unknown. HYQVIA should be given to a pregnant woman only if clearly indicated.
8.2 Lactation
Risk Summary
No human data are available to indicate the presence or absence of drug-associated risk. In animal studies, maternal antibodies binding to Recombinant Human Hyaluronidase were transferred to offspring during lactation. No adverse effects on pregnancy or offspring development were associated with anti-rHuPH20 antibodies. The effects of antibodies that bind to Recombinant Human Hyaluronidase of HYQVIA transferred during human lactation are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for HYQVIA and any potential adverse effects on the breastfed infant from HYQVIA or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Risk Summary
Animal studies do not indicate direct or indirect harmful effects of Recombinant Human Hyaluronidase with respect to reproductive potential at the doses used for facilitating administration of IG 10%. [See Carcinogenesis, Mutagenesis, Impairment of Fertility (13.1)]
-
11 DESCRIPTION
HYQVIA is a dual vial unit with one vial of Immune Globulin Infusion 10% (Human) and one vial of Recombinant Human Hyaluronidase.
The Immune Globulin Infusion 10% (Human) of HYQVIA is a ready-for-use, sterile, liquid preparation of highly purified and concentrated IgG antibodies. The distribution of the IgG subclasses is similar to that of normal plasma. The Fc and Fab functions are maintained in the primary component. Pre-kallikrein activator activity is not detectable. The Immune Globulin Infusion 10% (Human) of HYQVIA contains 100 mg/mL protein. At least 98% of the protein is IgG, average immunoglobulin A (IgA) concentration is 37μg/mL, and immunoglobulin M (IgM) is present in trace amounts. The Immune Globulin Infusion 10% (Human) of HYQVIA contains a broad spectrum of IgG antibodies against bacterial and viral agents. Glycine (0.25M) serves as a stabilizing and buffering agent. There is no added sugar, sodium, or preservatives. The pH is 4.6 to 5.1. The osmolality is 240 to 300 mOsmol/kg.
The Immune Globulin Infusion 10% (Human) of HYQVIA is manufactured from large pools of human plasma. IgG preparations are purified from plasma pools using a modified Cohn‑Oncley cold ethanol fractionation process, as well as cation and anion exchange chromatography.
Screening against potentially infectious agents begins with the donor selection process and continues throughout plasma collection and plasma preparation. Each individual plasma donation used in the manufacture of the Immune Globulin Infusion 10% (Human) of HYQVIA is collected only at FDA approved blood establishments and is tested by FDA licensed serological tests for Hepatitis B Surface Antigen (HBsAg), and for antibodies to Human Immunodeficiency Virus (HIV-1/HIV-2) and Hepatitis C Virus (HCV) in accordance with U.S. regulatory requirements. As an additional safety measure, mini-pools of the plasma are tested for the presence of HIV-1 and HCV by FDA licensed Nucleic Acid Testing (NAT).
To further improve the margin of safety, three dedicated, independent and effective virus inactivation/removal steps have been integrated into the manufacturing and formulation processes, namely solvent/detergent (S/D) treatment, 35 nm nanofiltration,and a low pH incubation at elevated temperature. The S/D process includes treatment with an organic mixture of tri‑n-butyl phosphate, octoxynol 9 and polysorbate 80 at 18°C to 25°C for a minimum of 60 minutes7.
In vitro virus spiking studies have been used to validate the capability of the manufacturing process to inactivate and remove viruses. To establish the minimum applicable virus clearance capacity of the manufacturing process, these virus clearance studies were performed under extreme conditions (e.g., at minimum S/D concentrations, incubation time and temperature for the S/D treatment). Virus clearance studies for the Immune Globulin Infusion 10% (Human) of HYQVIA performed in accordance with good laboratory practices (Table 6) have demonstrated that:
- S/D treatment inactivates the lipid-enveloped viruses investigated to below detection limits within minutes.
- 35 nm nanofiltration removes lipid-enveloped viruses to below detection limits and reduces the non‑lipid enveloped viruses HAV and B19V. As determined by a polymerase chain reaction assay, nanofiltration reduced B19V by a mean log10 reduction factor of 4.8 genome equivalents.
- Treatment with low pH at elevated temperature of 30°C to 32°C inactivates lipid-enveloped viruses and encephalomyocarditis virus (EMCV, model for HAV) to below detection limits, and reduces mice minute virus (MMV, model for B19V).
Table 6 Three Dedicated Independent Virus Inactivation/Removal Steps Mean Log10 Reduction Factors* (RFs) For Each Virus and Manufacturing Step Virus type Enveloped
RNAEnveloped DNA Non-enveloped
RNANon-enveloped
DNAFamily Retroviridae Flaviviridae Herpesviridae Picornaviridae Parvoviridae Virus HIV-1 BVDV WNV PRV HAV EMCV MMV Abbreviations: HIV-1, Human Immunodeficiency Virus Type 1; BVDV, Bovine Viral Diarrhea Virus (model for Hepatitis C Virus and other lipid enveloped RNA viruses); WNV, West Nile Virus; PRV, Pseudorabies Virus (model for lipid enveloped DNA viruses, including Hepatitis B Virus); EMCV, Encephalomyocarditis Virus (model for non-lipid enveloped RNA viruses, including Hepatitis A virus [HAV]); MMV, Mice Minute Virus (model for non-lipid enveloped DNA viruses, including B19 virus [B19V]); n.d. (not done), n.a. (not applicable). - * For the calculation of these RF data from virus clearance study reports, applicable manufacturing conditions were used. Log10 RFs on the order of 4 or more are considered effective for virus clearance in accordance with the Committee for Medicinal Products for Human Use (CHMP, formerly CPMP) guidelines.
- † No RF obtained due to immediate neutralization of HAV by the anti-HAV antibodies present in the product.
SD treatment
> 4.5
> 6.2
n.a.
> 4.8
n.d.
n.d.
n.d
35 nm nanofiltration
> 4.5
> 5.1
> 6.2
> 5.6
5.7
1.4
2.0
Low pH treatment
> 5.8
> 5.5
> 6.0
> 6.5
n.d. †
> 6.3
3.1
Overall log reduction factor (ORF)
> 14.8
> 16.8
> 12.2
> 16.9
5.7 †
> 7.7
5.1
The Recombinant Human Hyaluronidase of HYQVIA is produced from genetically engineered Chinese Hamster Ovary (CHO) cells containing a DNA plasmid encoding for a soluble fragment of human hyaluronidase PH20. The purified hyaluronidase glycoprotein contains 447 amino acids with an approximate molecular weight of 61,000 Daltons [see Mechanism of Action (12.1)]. This component is supplied as a sterile, clear, colorless, ready-for-use solution and has an approximate pH of 7.4 and an osmolality of 290 to 350 mOsm. Each vial contains 160 U/mL of Recombinant Human Hyaluronidase with 8.5 mg/mL sodium chloride, 1.78 mg/mL sodium phosphate dibasic dihydrate, 1.0 mg/mL human albumin, 1.0 mg/mL edentate disodium dihydrate, 0.40 mg/mL calcium chloride dihydrate, and 0.17 mg/mL sodium hydroxide added for pH adjustment. It does not contain preservatives.
Due to comprehensive virus testing at the Master Cell Bank, Working Cell Bank and bulk harvest stage, effective virus reduction during the purification process and use of pharmaceutical grade human albumin as an excipient with no other materials of human or animal origin involved in the manufacturing process, Recombinant Human Hyaluronidase provides for high margins of safety with respect to viruses.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The Immune Globulin Infusion 10% (Human) provides the therapeutic effect of HYQVIA. The Recombinant Human Hyaluronidase of HYQVIA increases dispersion and absorption of the Immune Globulin Infusion 10% (Human). The Immune Globulin Infusion 10% (Human) of HYQVIA supplies a broad spectrum of opsonizing and neutralizing IgG antibodies against a wide variety of bacterial and viral agents. The Immune Globulin Infusion 10% (Human) also contains a spectrum of antibodies capable of interacting with and altering the activity of cells of the immune system as well as antibodies capable of reacting with cells such as erythrocytes. The role of these antibodies and the mechanisms of action of IgG in the Immune Globulin Infusion 10% (Human) of HYQVIA have not been fully elucidated.
Hyaluronan is a polysaccharide found in the extracellular matrix of connective tissue8. It is depolymerized by the naturally occurring enzyme hyaluronidase. Unlike the stable structural components of the interstitial matrix, hyaluronan has a very fast turnover with a half-life of approximately 0.5 days. The Recombinant Human Hyaluronidase of HYQVIA increases permeability of the subcutaneous tissue by temporarily depolymerizing hyaluronan.In the doses administered, Recombinant Human Hyaluronidase of HYQVIA acts locally. The effects of the hyaluronidase are reversible and permeability of the subcutaneous tissue is restored within 24 to 48 hours.
12.3 Pharmacokinetics
The pharmacokinetics (PK) of HYQVIA was evaluated during a clinical trial of adults with PI after they achieved steady state at their 3 or 4 week dosing interval and underwent individual dose adjustment [see Clinical Studies (14)]. For adults, dose adjustment was based on a comparison of the ratios of the area under the IgG concentration versus time curve (AUC) during intravenous treatment versus during HYQVIA treatment.
The AUC of HYQVIA compared to conventional IGSC administration was 20% higher. The absolute bioavailability of HYQVIA was 93.3% relative to IGIV.
The pharmacokinetic parameters of HYQVIA compared with intravenously administered Immune Globulin Infusion 10% (Human) are shown in Table 7. The mean IgG dose in weekly equivalents was 147 mg/kg ± 50 (range 134 to 160 mg/kg). The serum IgG trough levels are comparable: mean serum IgG trough with HYQVIA was 1077 mg/dL compared with 1095 mg/dL with intravenously administered Immune Globulin Infusion 10% (Human). C max was lower with HYQVIA (1607 mg/dL) than with intravenously administered Immune Globulin Infusion 10% (Human) (2248 mg/dL). Time to reach maximum concentration of IgG following HYQVIA administration was 5 (3.3-5.1) days.
In the extension trial, reducing the dosing interval from 4 to 2 weeks resulted in a mean increase of 13% in serum IG trough levels.
Table 7 Pharmacokinetic Parameters of HYQVIA Compared to Intravenously Administered Immune Globulin Infusion 10% (Human) (IGIV) - * N=58 for HYQVIA and N=67 for IGIV
- † Standardized to a 7 day interval
- ‡ N=58 for HYQVIA
- § Apparent clearance
HYQVIA
IGIV
Number of Subjects
60
68
IgG Weekly Dose [mg/kg/week]
Mean (SD)
147 (50)
139 (55)
95% CI
134 to 160
126 to 153
C max [mg/dL]
Mean (SD)
1607 (382)
2248 (547)
95% CI
1508 to 1706
2116 to 2380
IgG Trough Levels[mg/dL]*
Mean (SD)
1077 (275)
1095 (321)
95% CI
1004 to 1149
1017 to 1174
AUC/week [g*days/L]†
Mean (SD)
91.4 (21)
98.7 (24.3)
95% CI
85.9 to 96.8
92.8 to 104.5
Bioavailability‡
Point estimate
93.3
100% (defined)
90% CI
91.4 to 95.2
N/A
Clearance [mL/kg/day]
Mean (SD)
1.6 (0.5)§
1.4 (0.4)
95% CI
1.5 to 1.8
1.3 to 1.5
Terminal Half-life [days]
Mean (SD)
59.3 (36.1)
41.6 (26.9)
95% CI
50 to 68.6
35.1 to 48.1
T max [days]
Median
5.0
0.1
95% CI
3.3 to 5.1
0.1 to 0.1
Figure 1 shows a mean concentration-time plot of IgG in subjects 12 years and older. The concentration-time profile of HYQVIA is similar to that of intravenous administration but without the high peak. The peak to trough variation is more similar to subcutaneous administration.
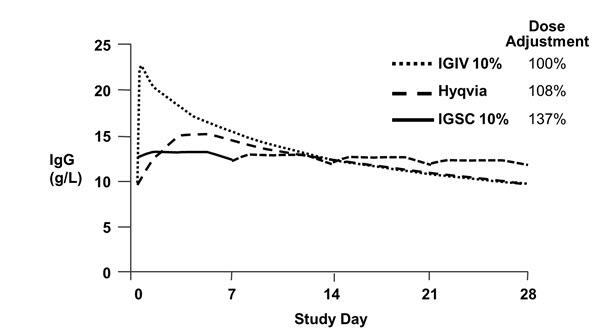
Figure 1 Pharmacokinetic Comparison of Mean IgG Values for HYQVIA vs. Intravenously and Subcutaneously Administered Immune Globulin Infusion 10% (Human)*
* IGIV and HYQVIA data at 28 day dosing interval; IGSC data at 7 day dosing interval; IGSC dotted line shows weekly dose extrapolated over 21 additional days.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Immune Globulin Infusion 10% (Human)
Long-term animal studies have not been conducted to evaluate the carcinogenic potential of Immune Globulin Infusion 10% (Human) or its effect on fertility.
An in vitro mutagenicity test was performed for Immune Globulin Infusion 10% (Human). No evidence of mutagenicity was observed.
Recombinant Human Hyaluronidase
Hyaluronidases are found in most tissues of the body. Long-term animal studies to evaluate the carcinogenic or mutagenic potential of Recombinant Human Hyaluronidase have not been conducted.
No adverse effects on fertility were observed in mice, rabbits and cynomolgus monkeys exposed to antibodies that bind to Recombinant Human Hyaluronidase and species-specific hyaluronidase. Reversible infertility has been observed in male and female guinea pigs immunized to produce antibodies to hyaluronidase. However, antibodies to hyaluronidase did not influence reproduction following immunization of mice, rabbits, sheep, or cynomolgus monkeys. The effects of antibodies that bind to Recombinant Human Hyaluronidase on human fertility are unknown.
13.2 Animal Toxicology and/or Pharmacology
Developmental studies in mice demonstrated that administration of Recombinant Human Hyaluronidase did not produce teratogenicity or signs of maternal toxicity at doses up to 18 mg/kg (2.2 x 106 U/kg), which is 28,800 times higher than the typical monthly human dose. Maternal doses of 9 and 18 mg/kg were associated with reduced fetal weight and an increased number of fetal resorptions. No adverse effects on fetal development were observed at a maternal dose of 3 mg/kg (360,000 U/kg), which is 4800 times higher than the typical monthly human dose.
In a peri-and post-natal reproduction study, female mice were dosed daily with Recombinant Human Hyaluronidase from implantation through lactation and weaning. There were no adverse effects on gestation, parturition, lactation and maternal behavior or on the development of the male or female offspring of the treated female mice in terms of sexual maturation, learning and memory of offspring, or their ability to produce another generation of offspring at doses up to 9 mg/kg (1.1 x106 Unit/kg), which is 14,400 times higher than the typical monthly human dose.
-
14 CLINICAL STUDIES
A prospective, open-label, non-controlled, multi-center trial was conducted in the US to determine the efficacy, tolerability and pharmacokinetics (PK) of HYQVIA in subjects with PI. Two cohorts of subjects were enrolled. Thirty-one subjects had been treated intravenously for three months and then subcutaneously each week at 137% of the intravenous dose for approximately one year before transitioning to the HYQVIA trial. The remaining subjects also were treated intravenously for 3 months and then immediately began treatment with HYQVIA in the trial.
One week after the last intravenous or subcutaneous infusion, each subject began subcutaneous treatment with HYQVIA. After placing the subcutaneous needle set, the Recombinant Human Hyaluronidase of HYQVIA was infused through the needle set followed within 10 minutes by the immune globulin of HYQVIA at 108% of the intravenous dose. Dosing began with a 1-week equivalent dose. One week later, a 2-week dose was administered, followed 2 weeks later with a 3-week dose. For those subjects who were on a 4-week dose interval prior to entering the trial, 3 weeks later the 4-week dose was administered. This ramp-up period allowed subjects to become familiar with the large volumes required for a full 3- or 4-week treatment. Subsequently, subjects continued the 3- or 4-week dosing for the remainder of the trial. After 3 doses at the full volume, a serum IgG trough level was obtained for all subjects and used to individually adapt the subcutaneous dose of HYQVIA to compensate for individual variation from the mean value of 108% [see Pharmacokinetics (12.3) and Dosage and Administration (2.1)]. All subjects who completed the trial received a minimum of 12 infusions at this individually adapted dose. The period after the ramp-up was considered the efficacy period and used for safety and efficacy analyses.
Outcome measures included the rate of infections, adverse reactions, tolerability of the infusions of HYQVIA, number of infusion sites per month, and infusion rate. Eighty-nine subjects were enrolled, 87 treated intravenously and 83 treated with HYQVIA. The majority were Caucasian (79/87, 90.8%). Median age was 35.0 years (range 4 to 78 years). Forty-four of the subjects were naïve to subcutaneous treatment. Median serum IgG trough levels for the 6 months before enrollment were 1033.5 mg/dL (range: 405 to 3200 mg/dL) in subcutaneous-experienced subjects and 1000 mg/dL (range: 636 to 3200) in the subcutaneous-naïve subjects.
The 83 subjects received a total of 1359 infusions of HYQVIA during the entire trial. Of these, 1129 were administered after the ramp-up when the subjects were on a consistent interval of 3 or 4 weeks, which was predetermined to be the efficacy period for data analysis.
Median duration of treatment in the IGIV period was 91 days (range 84 to 122 days). Median duration of HYQVIA treatment during the dose ramp up period was 42 days (range 20 to 49), and during the efficacy period was 366 days (range 42 to 507 days). None of the subjects withdrew due to a severe or serious local or systemic adverse reaction [see Clinical Trial Experience (6.1)].
There were two acute serious bacterial infections (ASBI), both of which were episodes of pneumonia treated as outpatients with oral antibiotics during the 12-month efficacy period; an additional pneumonia requiring hospitalization occurred during the ramp-up. Based on this, the annualized rate of ASBI while treated with HYQVIA was 0.025, with an upper 99% confidence limit of 0.046, which is significantly less than (p < 0.0001) the rate of one infection per year10.
The overall rates of infections throughout both the efficacy and extension trials are shown in Table 8. The secondary endpoints evaluated in the efficacy trial were the annual rate of all infections and other efficacy measures.
Table 8 Summary of Infections and Other Secondary Efficacy Endpoints Annual Rate
Parameter
Mean
95% CI
Infections per patient per year (Efficacy Trial)
2.97
2.51 to 3.47
Infections per patient per year (Efficacy and Extension Trials)
2.99
2.60 to 3.92
Days off school/work
3.41
2.44 to 4.5
Days on antibiotics
20.58
15.71 to 26.3
Unscheduled physician visits for infections
4.87
3.9 to 5.97
Days in hospital due to infection
0.0
0.0 to 0.12
An objective of the trial was to achieve the same number or fewer infusions with HYQVIA per month as with intravenous administration and significantly fewer than with conventional subcutaneous administration. A summary of intravenous administration compared with HYQVIA administration is presented in Table 9.
Table 9 Summary of Infusions Parameter
Intravenous
HYQVIA
Median monthly number of infusion sites
1.34
(1.2 to 1.7)
1.09
(1.0 to 3.5)
Mean volume per site (mL)
339
(75 to 800)
292
(91 to 648)
Dose per site (g)
33.9
(7.5 to 80.0)
29.2
(9.1 to 64.8)
Median duration of individual infusions (hr)
2.33
(0.92 to 6.33)
2.08
(0.83 to 4.68)
Monthly median infusion time (hr/month)
3.2
2.64
Median maximum infusion rate (mL/hr)
246
(60 to 668)
300
(10 to 300)
Percent (%) of infusion completed
without change in rate, interruption
and discontinuation
95.9
97.7
Sixteen of 83 subjects (19.3%) were infused every 3 weeks and 67 (80.7%) were infused every 4 weeks. Seventy-eight of 83 (94%) subjects attained the same 3- or 4-week dosing as their previous IV treatment. One decreased from 4 to 3 weeks, one from 4 to 2 weeks and one from 3 to 2 weeks. The primary reason for decreasing the interval was discomfort due to swelling.
In a separate study evaluating subcutaneous treatment with Immune Globulin Infusion 10% (Human), a median of 21.43 sites were required each month with a median monthly infusion time of 5.35 hours.
-
15 REFERENCES
- 1. Orange JS, Hossny EM, Weiler CR, Ballow M, Berger M, Bonilla FA, Buckley R, Chinen J, El-Gamal Y, Mazer BD, Nelson Jr. RP, Patel DD, Secord E, Sorenson RU, Wasserman RL, Cunningham-Rundles C, Use of Intravenous Immunoglobulin in Human Disease: A Review of Evidence by Members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma, and Immunology. J Allergy Clin Immunol 2006; 117:S525-53.
- 2. Bonilla FA, Bernstein IL, Khan DA, Ballas ZK, Chinen J, Frank MM, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol. 2005; 94(suppl 1):S1-63.
- 3. Eijkhout HW, Der Meer JW, Kallenberg CG, et al. The effect of two different dosages of intravenous immunoglobulin on the incidence of recurrent infections in patients with primary hypogammaglobulinemia. A randomized, double-blind, multicenter crossover trial. Ann Intern Med. 2001;135:165-174.
- 4. Pierce LR, Jain N. Risks associated with the use of intravenous immunoglobulin. Transfusion Med Rev. 2003;17:241-251.
- 5. Katz U, Sheonfeld Y. Review: intravenous immunoglobulin therapy and thromboembolic complications. Lupus 2005;14:802-8
- 6. Daw Z, Padmore R, Neurath D, Cober N, Tokessy M, Desjardins D, et al. Hemolytic transfusion reactions after administration of intravenous intravenous immune (gamma) globulin: a case series analysis. Transfusion 2008; 48:1598-601
- 7. Kreil TR, Berting A, Kistner O, Kindermann J. West Nile virus and the safety of plasma derivatives: verification of high safety margins, and the validity of predictions based on model virus data. Transfusion 2003;43:1023-1028.
- 8. Bookbinder LH, Hofer A, Haller MF, Zepeda ML, Keller G-A, Lim JE, Edginton TS, Shepard HM, Patton JS, Frost GI. A recombinant human enzyme for enhanced interstitial transport of therapeutics. J of Controlled Release 2006; 114:230-241.
- 9. Wasserman RL, Melamed I, Kobrynski L, Strausbaugh SD, Stein MR, Sharkhawy M, Engl W, Leibl H, Sobolevsky L, Gelmont D, Schiff RI, Grossman WJ. Efficacy, Safety, and Pharmacokinetics of a 10% Liquid Immune Globulin Preparation (GAMMAGARD LIQUID, 10%) Administered Subcutaneously in Subjects with Primary Immunodeficiency Disease. J Clin Immunol. 2011 Mar 22. [Epub ahead of print]
- 10. Golding B. IGIV Clinical Endpoints. Presented at: Blood Products Advisory Committee, 65th Meeting. 17 March 2000. Silver Spring, MD.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
HYQVIA is supplied in a dual vial unit of two single use vials containing the labeled amount of functionally active Immune Globulin Infusion 10% (Human) and Recombinant Human Hyaluronidase. The packaging of this product is not made with natural rubber latex.
The following presentations of HYQVIA are available:
Immune Globulin Infusion 10% (Human)
Recombinant Human Hyaluronidase
NDC Number
Volume
Grams Protein
Volume
Units
0944-2510-02
25 mL
2.5
1.25 mL
200
0944-2511-02
50 mL
5.0
2.5 mL
400
0944-2512-02
100 mL
10.0
5.0 mL
800
0944-2513-02
200 mL
20.0
10.0 mL
1600
0944-2514-02
300 mL
30.0
15.0 mL
2400
Storage and Handling
Do not freeze.
Keep the vials in the carton in order to protect from light.
Refrigeration: 2° to 8°C [36° to 46°F] for up to 36 months.
Room Temperature: up to 25°C [77°F] for up to 3 months during the first 24 months from the date of manufacturing (Mfg date) printed on the carton.
- HYQVIA must be used within 3 months after removal to room temperature but within the expiration date on the carton and vial label.
- Do not return HYQVIA to the refrigerator after it has been stored at room temperature.
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Information for Patients).
Inform patients to immediately report the following signs and symptoms to their healthcare professional:
- Acute respiratory distress, wheezing, swelling of the airway or severe hives or itching. [see Warnings and Precautions (5.1)]
- Instruct patients to immediately report symptoms of thrombosis. These symptoms may include pain and/or swelling of an arm or leg with warmth over the affected area, discoloration of an arm or leg, unexplained shortness of breath, chest pain or discomfort that worsens on deep breathing, unexplained rapid pulse, numbness or weakness on one side of the body. [see Warnings and Precautions (5.2)]
- Advise patients that PH20 antibodies can develop. The potential exists for such antibodies to cross-react with endogenous PH20, which is known to be expressed in the adult male reproductive tract. The clinical significance of these antibodies is unknown. [see Warning and Precautions (5.3)]
- Severe headache, neck stiffness, drowsiness, fever, sensitivity to light, painful eye movements, nausea, and vomiting. [see Warnings and Precautions (5.3)]
- Increased heart rate, fatigue, yellowing of the skin or eyes, and dark-colored urine. [see Warnings and Precautions (5.5)]
- Decreased urine output, sudden weight gain, fluid retention/edema, and/or shortness of breath. [see Warnings and Precautions (5.6)]
- Trouble breathing, chest pain, blue lips or extremities, or fever that can occur 1 to 6 hours after an infusion of HYQVIA. [see Warnings and Precautions (5.8)]
- Inform patients that HYQVIA is made from human plasma and may contain infectious agents that can cause disease (e.g., viruses and, theoretically, the vCJD agent). Patients should report any symptoms that concern them which might be caused by virus infections. [see Warnings and Precautions (5.9)]
- Inform the female patient of the possibility of participating in the pregnancy registry. [see Use in Specific Populations (8.1)]
- Inform patients that HYQVIA can interfere with their immune response to live viral vaccines such as measles, mumps, rubella, and varicella, and instruct patients to notify their healthcare professional of this potential interaction when they are receiving vaccinations [see Drug Interactions (7)].
Self-administration – If self-administration is deemed appropriate by the physician, give clear instructions and training on how to administer HYQVIA. Document their ability to independently administer HYQVIA.
- Ensure the patient understands the importance of following regularly scheduled infusions to maintain appropriate steady IgG levels.
- Instruct the patient to keep a treatment infusion log. This infusion log should include information about each infusion such as, the lot number(s), infusion site location, the time, date, dose, and any reactions.
- Inform the patient that due to the volume that can be infused, swelling is common with HYQVIA. Mild to moderate local infusion-site reactions (e.g., swelling and redness) are common side effects of facilitated subcutaneous treatment with HYQVIA. Instruct the patient to contact their healthcare professional if a local reaction increases in severity or persists for more than a few days.
- Instruct the patient on the importance of following the directions for the pump for infusion of the Immune Globulin Infusion 10% (Human) of HYQVIA.
BAXALTA® and HYQVIA® are trademarks of Baxalta Incorporated, a Takeda company. TAKEDA and the TAKEDA logo are trademarks or registered trademarks of Takeda Pharmaceutical Company Limited.
Patented: please see https://www.shire.com/legal-notice/product-patents
Baxalta US Inc.
Lexington, MA 02421 USA
U.S. License No. 202017H011-HYQ-US
-
Information For Patients
HYQVIA
Immune Globulin Infusion 10% (Human)
With Recombinant Human Hyaluronidase
For Subcutaneous Administration
The following summarizes important information about HYQVIA (pronounced Hi-Q-via). Please read it carefully before using this medicine. This information does not take the place of talking with your healthcare professional, and it does not include all of the important information about HYQVIA. If you have any questions after reading this, ask your healthcare professional.
What is the most important information that I should know about HYQVIA?
- HYQVIA can cause blood clots.
- Call your healthcare professional if you have pain, swelling, warmth, redness, or a lump in your legs or arms, other than at the infusion site(s), unexplained shortness of breath, chest pain or discomfort that worsens on deep breathing, unexplained rapid pulse, numbness or weakness on one side of the body.
- Your healthcare professional may perform blood tests regularly to check your IgG level.
- With your consent, your healthcare professional may provide blood samples to Baxalta US Inc. to test for antibodies that may form against the hyaluronidase part of HYQVIA.
- Do not infuse HYQVIA into or around an infected or red swollen area because it can cause infection to spread.
What should I tell my healthcare professional before I start using HYQVIA?
Before starting HYQVIA, tell your healthcare professional if you:
- Have or had any kidney, liver, or heart problems or history of blood clots because HYQVIA can make these problems worse.
- Have IgA deficiency or a history of severe allergic reactions to IgG or other blood products
- Are pregnant, trying to become pregnant or are breast feeding.
What is HYQVIA?
HYQVIA is a liquid medicine containing immune globulin and Recombinant Human Hyaluronidase. HYQVIA contains IgG antibodies, collected from human plasma donated by healthy people. The antibodies help your body to fight off bacterial and viral infections. The hyaluronidase part of HYQVIA helps more of the immune globulin get absorbed into the body to fight infection.
Who should not take HYQVIA?
- Do not take HYQVIA if you: Are allergic to IgG, hyaluronidase, other blood products, or any ingredient in HYQVIA.
How should I take HYQVIA?
- HYQVIA is infused under the skin (subcutaneously) up to once every 4 weeks.
- You can get HYQVIA at your healthcare professional's office, clinic, or hospital.
- You can use HYQVIA at home. You and your healthcare professional will decide if home self-infusion is right for you.
What are the possible or reasonably likely side effects of HYQVIA?
After HYQVIA infusion a temporary, soft swelling may occur around the infusion site, which may last 1 to 3 days, due to the volume of fluid infused.
The following local reactions may occur at the site of infusion and generally go away in a few hours. Local reactions are less likely after the first few infusions.
Mild or moderate pain
Redness
Swelling
Itching
The most common side effects of HYQVIA are:
Headache
Vomiting
Fatigue
Nausea
Fever
Antibodies to the hyaluronidase component of HYQVIA were formed in some patients taking HYQVIA. It is not known if there is any long term effect. In theory, these antibodies could react with your body's own PH20. PH20 is present in the male reproductive tract. So far, these antibodies have not been associated with increased or new side-effects.
Call your healthcare professional or go to your emergency department right away if you get:
- Hives, swelling in the mouth or throat, itching, trouble breathing, wheezing, fainting or dizziness. These could be signs of a serious allergic reaction.
- Bad headache with nausea, vomiting, stiff neck, fever, and sensitivity to light. These could be signs of swelling in your brain.
- Reduced urination, sudden weight gain, or swelling in your legs. These could be signs of a kidney problem.
- Pain, swelling, warmth, redness, or a lump in your legs or arms, other than at the infusion site(s). These could be signs of a blood clot.
- Brown or red urine, fast heart rate, yellow skin or eyes. These could be signs of a liver or blood problem.
- Chest pain or trouble breathing, blue lips or extremities. These could be signs of a lung problem.
These are not all of the possible side effects for HYQVIA. You can ask your healthcare professional for information that is provided to healthcare professionals. Talk to your healthcare professional about any side effects that bother you or that don't go away.
How do I store HYQVIA?
Store HYQVIA refrigerated or at room temperature.
- You can store HYQVIA in the refrigerator (36° to 46°F [2° to 8°C]) for up to 36 months.
- You can store HYQVIA at room temperature (up to 77°F [25°C]) for up to 3 months during the first 24 months from the date of manufacturing (Mfg Date) printed on the carton.
- Do not return HYQVIA to the refrigerator if you take it out to room temperature.
Check the expiration date on the carton and vial label. Do not use HYQVIA after the expiration date.
Do not freeze.
Protect from light. You can use the original HYQVIA containers to protect it from light.
Resources at Baxalta Available to the Patients
For more information on patient resources and education, please visit www.immunedisease.com.
Detailed Instructions for Administration
Do not use HYQVIA at home until you get instructions and training from your healthcare professional.
Your healthcare professional will decide which administration system that is right for you. You will take the hyaluronidase first. Then, within 10 minutes, you will take the immune globulin through an infusion pump.
Prepare HYQVIA vial(s):
- Remove HYQVIA from the box. Allow vials to reach room temperature. This may take up to 60 minutes.
- Do not apply heat or place in microwave.
- Do not shake the vials.
BAXALTA® and HYQVIA® are trademarks of Baxalta Incorporated, a Takeda company. TAKEDA and the TAKEDA logo are trademarks or registered trademarks of Takeda Pharmaceutical Company Limited.
Patented: please see https://www.shire.com/legal-notice/product-patents
Baxalta US Inc.
Lexington, MA 02421 USA
U.S. License No. 2020Issue Date: 2/2020
17H011-HYQ-US
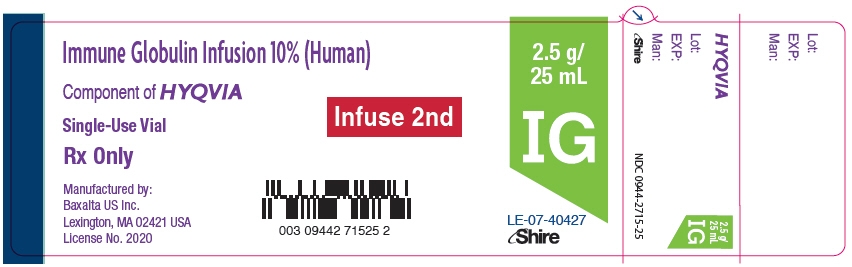
- PRINCIPAL DISPLAY PANEL - 2.5 g/25 mL Vial Label
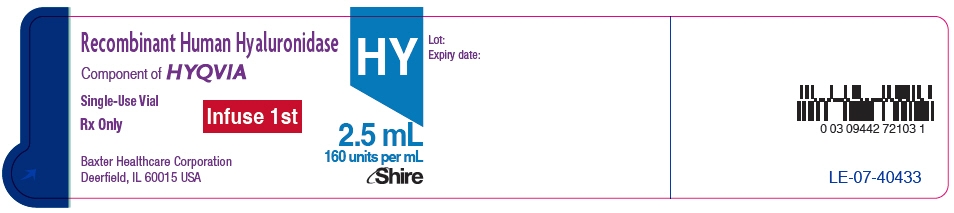
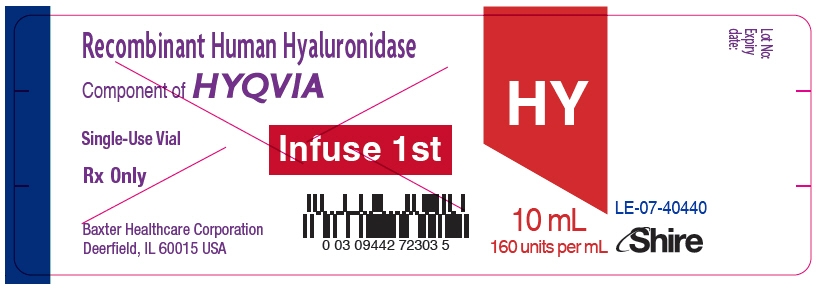
- PRINCIPAL DISPLAY PANEL - 1.25 mL Vial Label
-
PRINCIPAL DISPLAY PANEL - 2.5 g/25 mL Kit Carton
NDC: 0944-2510-02
Immune Globulin Infusion 10% (Human)
with Recombinant Human Hyaluronidase
HYQVIA2.5 g/
25 mLFor Subcutaneous Administration Only.
Single-use dual vial unit
Directions for use:
Read the enclosed Package Insert.No preservative
Rx Only
Shire
- PRINCIPAL DISPLAY PANEL - 5 g/50 mL Vial Label
- PRINCIPAL DISPLAY PANEL - 2.5 mL Vial Label
-
PRINCIPAL DISPLAY PANEL - 5 g/50 mL Kit Carton
NDC: 0944-2511-02
Immune Globulin Infusion 10% (Human)
with Recombinant Human Hyaluronidase
HYQVIA5 g/
50 mLFor Subcutaneous Administration Only.
Single-use dual vial unit
Directions for use:
Read the enclosed Package Insert.No preservative
Rx Only
Shire
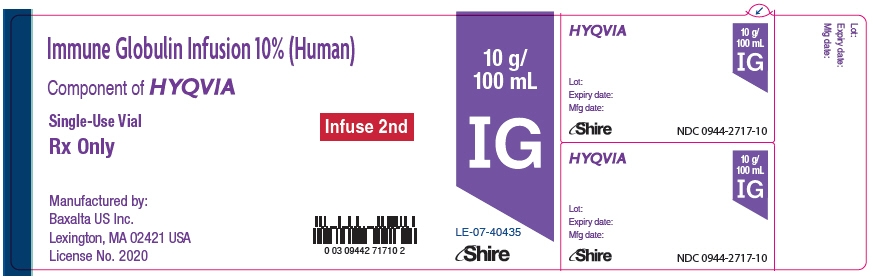
- PRINCIPAL DISPLAY PANEL - 10 g/100 mL Vial Label
- PRINCIPAL DISPLAY PANEL - 5 mL Vial Label
-
PRINCIPAL DISPLAY PANEL - 10 g/100 mL Kit Carton
NDC: 0944-2512-02
Immune Globulin Infusion 10% (Human)
with Recombinant Human Hyaluronidase
HYQVIA10 g/
100 mLFor Subcutaneous Administration Only.
Single-use dual vial unit
Directions for use:
Read the enclosed Package Insert.No preservative
Rx Only
Shire
- PRINCIPAL DISPLAY PANEL - 20 g/200 mL Vial Label
- PRINCIPAL DISPLAY PANEL - 10 mL Vial Label
-
PRINCIPAL DISPLAY PANEL - 20 g/200 mL Kit Carton
NDC: 0944-2513-02
Immune Globulin Infusion 10% (Human)
with Recombinant Human Hyaluronidase
HYQVIA20 g/
200 mLFor Subcutaneous Administration Only.
Single-use dual vial unit
Directions for use: Read the enclosed Package Insert.
No preservative
Rx Only
Shire
- PRINCIPAL DISPLAY PANEL - 30 g/300 mL Vial Label
- PRINCIPAL DISPLAY PANEL - 15 mL Vial Label
-
PRINCIPAL DISPLAY PANEL - 30 g/300 mL Kit Carton
NDC: 0944-2514-02
Immune Globulin Infusion 10% (Human)
with Recombinant Human Hyaluronidase
HYQVIA30 g/
300 mLFor Subcutaneous Administration Only.
Single-use dual vial unit
Directions for use: Read the enclosed Package Insert.
No preservative
Rx Only
Shire
-
INGREDIENTS AND APPEARANCE
HYQVIA
immune globulin 10 percent (human) with recombinant human hyaluronidase kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 0944-2510 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-2510-02 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 25 mL Part 2 1 VIAL, GLASS 1.25 mL Part 1 of 2 IMMUNE GLOBULIN INFUSION (HUMAN), 10%
human immuneglobulin g solutionProduct Information Item Code (Source) NDC: 0944-2715 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN IMMUNOGLOBULIN G (UNII: 66Y330CJHS) (HUMAN IMMUNOGLOBULIN G - UNII:66Y330CJHS) HUMAN IMMUNOGLOBULIN G 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCINE (UNII: TE7660XO1C) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-2715-25 25 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 06/04/2012 Part 2 of 2 RECOMBINANT HUMAN HYALURONIDASE
hyaluronidase (human recombinant) solutionProduct Information Item Code (Source) NDC: 0944-2720 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYALURONIDASE (HUMAN RECOMBINANT) (UNII: 743QUY4VD8) (HYALURONIDASE (HUMAN RECOMBINANT) - UNII:743QUY4VD8) HYALURONIDASE (HUMAN RECOMBINANT) 160 [USP'U] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE (UNII: SE337SVY37) ALBUMIN HUMAN (UNII: ZIF514RVZR) EDETATE DISODIUM (UNII: 7FLD91C86K) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-2720-03 1.25 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 09/12/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 09/12/2014 HYQVIA
immune globulin 10 percent (human) with recombinant human hyaluronidase kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 0944-2511 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-2511-02 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 50 mL Part 2 1 VIAL, GLASS 2.5 mL Part 1 of 2 IMMUNE GLOBULIN INFUSION (HUMAN), 10%
human immuneglobulin g solutionProduct Information Item Code (Source) NDC: 0944-2716 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN IMMUNOGLOBULIN G (UNII: 66Y330CJHS) (HUMAN IMMUNOGLOBULIN G - UNII:66Y330CJHS) HUMAN IMMUNOGLOBULIN G 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCINE (UNII: TE7660XO1C) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-2716-05 50 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 06/04/2012 Part 2 of 2 RECOMBINANT HUMAN HYALURONIDASE
hyaluronidase (human recombinant) solutionProduct Information Item Code (Source) NDC: 0944-2721 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYALURONIDASE (HUMAN RECOMBINANT) (UNII: 743QUY4VD8) (HYALURONIDASE (HUMAN RECOMBINANT) - UNII:743QUY4VD8) HYALURONIDASE (HUMAN RECOMBINANT) 160 [USP'U] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE (UNII: SE337SVY37) ALBUMIN HUMAN (UNII: ZIF514RVZR) EDETATE DISODIUM (UNII: 7FLD91C86K) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-2721-03 2.5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 09/12/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 09/12/2014 HYQVIA
immune globulin 10 percent (human) with recombinant human hyaluronidase kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 0944-2512 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-2512-02 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 100 mL Part 2 1 VIAL, GLASS 5 mL Part 1 of 2 IMMUNE GLOBULIN INFUSION (HUMAN), 10%
human immuneglobulin g solutionProduct Information Item Code (Source) NDC: 0944-2717 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN IMMUNOGLOBULIN G (UNII: 66Y330CJHS) (HUMAN IMMUNOGLOBULIN G - UNII:66Y330CJHS) HUMAN IMMUNOGLOBULIN G 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCINE (UNII: TE7660XO1C) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-2717-10 100 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 06/04/2012 Part 2 of 2 RECOMBINANT HUMAN HYALURONIDASE
hyaluronidase (human recombinant) solutionProduct Information Item Code (Source) NDC: 0944-2722 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYALURONIDASE (HUMAN RECOMBINANT) (UNII: 743QUY4VD8) (HYALURONIDASE (HUMAN RECOMBINANT) - UNII:743QUY4VD8) HYALURONIDASE (HUMAN RECOMBINANT) 160 [USP'U] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE (UNII: SE337SVY37) ALBUMIN HUMAN (UNII: ZIF514RVZR) EDETATE DISODIUM (UNII: 7FLD91C86K) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-2722-03 5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 09/12/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 09/12/2014 HYQVIA
immune globulin 10 percent (human) with recombinant human hyaluronidase kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 0944-2513 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-2513-02 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 200 mL Part 2 1 VIAL, GLASS 10 mL Part 1 of 2 IMMUNE GLOBULIN INFUSION (HUMAN), 10%
human immuneglobulin g solutionProduct Information Item Code (Source) NDC: 0944-2718 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN IMMUNOGLOBULIN G (UNII: 66Y330CJHS) (HUMAN IMMUNOGLOBULIN G - UNII:66Y330CJHS) HUMAN IMMUNOGLOBULIN G 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCINE (UNII: TE7660XO1C) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-2718-20 200 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 06/04/2012 Part 2 of 2 RECOMBINANT HUMAN HYALURONIDASE
hyaluronidase (human recombinant) solutionProduct Information Item Code (Source) NDC: 0944-2723 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYALURONIDASE (HUMAN RECOMBINANT) (UNII: 743QUY4VD8) (HYALURONIDASE (HUMAN RECOMBINANT) - UNII:743QUY4VD8) HYALURONIDASE (HUMAN RECOMBINANT) 160 [USP'U] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE (UNII: SE337SVY37) ALBUMIN HUMAN (UNII: ZIF514RVZR) EDETATE DISODIUM (UNII: 7FLD91C86K) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-2723-03 10 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 09/12/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 09/12/2014 HYQVIA
immune globulin 10 percent (human) with recombinant human hyaluronidase kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 0944-2514 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-2514-02 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 300 mL Part 2 1 VIAL, GLASS 15 mL Part 1 of 2 IMMUNE GLOBULIN INFUSION (HUMAN), 10%
human immuneglobulin g solutionProduct Information Item Code (Source) NDC: 0944-2719 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN IMMUNOGLOBULIN G (UNII: 66Y330CJHS) (HUMAN IMMUNOGLOBULIN G - UNII:66Y330CJHS) HUMAN IMMUNOGLOBULIN G 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCINE (UNII: TE7660XO1C) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-2719-30 300 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 06/04/2012 Part 2 of 2 RECOMBINANT HUMAN HYALURONIDASE
hyaluronidase (human recombinant) solutionProduct Information Item Code (Source) NDC: 0944-2724 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYALURONIDASE (HUMAN RECOMBINANT) (UNII: 743QUY4VD8) (HYALURONIDASE (HUMAN RECOMBINANT) - UNII:743QUY4VD8) HYALURONIDASE (HUMAN RECOMBINANT) 160 [USP'U] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE (UNII: SE337SVY37) ALBUMIN HUMAN (UNII: ZIF514RVZR) EDETATE DISODIUM (UNII: 7FLD91C86K) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-2724-03 15 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 09/12/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 09/12/2014 Labeler - Baxalta US Inc. (079887619) Establishment Name Address ID/FEI Business Operations Baxalta Belgium Manufacturing SA 370634700 MANUFACTURE(0944-2510, 0944-2511, 0944-2512, 0944-2513, 0944-2514) Establishment Name Address ID/FEI Business Operations Baxter Pharmaceutical Solutions, LLC 604719430 MANUFACTURE(0944-2510, 0944-2511, 0944-2512, 0944-2513, 0944-2514)
Trademark Results [HYQVIA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HYQVIA 86348519 4774188 Live/Registered |
BAXALTA INCORPORATED 2014-07-25 |
 HYQVIA 85239796 4669391 Live/Registered |
BAXALTA INCORPORATED 2011-02-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.