HARLIKU- nitisinone tablet
HARLIKU by
Drug Labeling and Warnings
HARLIKU by is a Prescription medication manufactured, distributed, or labeled by Cycle Pharmaceuticals Ltd., Rivopharm SA, Penn Pharmaceutical Services Limited, Catalent Nottingham Limited, Pharmaron Manufacturing Services (UK) Limited, Central Pharma Contract Packing Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use HARLIKU safely and effectively. See full prescribing information for HARLIKU.
HARLIKUTM (nitisinone) tablets, for oral use
Initial U.S. Approval: 2002INDICATIONS AND USAGE
HARLIKU is a hydroxyphenyl-pyruvate dioxygenase inhibitor indicated for the reduction of urine homogentisic acid (HGA) in adult patients with alkaptonuria (AKU). (1)
DOSAGE AND ADMINISTRATION
The recommended dosage of HARLIKU is 2 mg administered orally, once daily. (2.1)
DOSAGE FORMS AND STRENGTHS
Tablets: 2 mg. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
Ocular Symptoms and Hyperkeratotic Plaques Due To Elevated Plasma Tyrosine Levels: Inadequate restriction of tyrosine and phenylalanine intake can lead to elevations in plasma tyrosine and levels above 500 micromol/L may lead to ocular signs and symptoms or painful hyperkeratotic plaques on the soles and palms. (5.1)
- Assess plasma tyrosine levels in patients presenting with ocular signs and symptoms. (5.1)
- Obtain slit-lamp examination prior to treatment and regularly thereafter. (5.1)
- Implement diet restriction and/or treatment interruption as appropriate. (5.1)
Leukopenia and Severe Thrombocytopenia: Monitor platelet and white blood cell counts. (5.2)
ADVERSE REACTIONS
Most common adverse reactions (>1%) are elevated tyrosine levels, keratitis and thrombocytopenia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Cycle Pharmaceuticals Ltd at 1-855-831-5413 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
Sensitive CYP2C9 Substrates: Reduce dosage of co-administered drug metabolized by CYP2C9 by half. (7.1)
OAT1/OAT3 Substrates: Avoid concomitant use of HARLIKU with OAT1/OAT3 substrates. Concomitant use with OAT1/OAT3 substrates may increase the risk of adverse reactions related to the co-administered drug. (7.1)See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Ocular Symptoms and Hyperkeratotic Plaques Due to Elevated Plasma Tyrosine Levels
5.2 Leukopenia and Severe Thrombocytopenia
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effects of HARLIKU on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage of HARLIKU is 2 mg administered orally, once daily.
Administer HARLIKU with or without food [see Clinical Pharmacology (12.3)].
Missed Dose
If a dose of HARLIKU is missed, do not administer two doses at once to make up for a missed dose. Take the next dose at the scheduled time. - 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Ocular Symptoms and Hyperkeratotic Plaques Due to Elevated Plasma Tyrosine Levels
Treatment with HARLIKU may cause elevated plasma tyrosine levels in patients with AKU. Tyrosine levels greater than 500 micromol/L may lead to the following:
- Ocular signs and symptoms including keratitis, corneal opacities, corneal irritation, corneal ulcers, conjunctivitis, eye pain, and photophobia. These ocular adverse reactions have been reported in patients treated with nitisinone [see Adverse Reactions (6.1)]. In a clinical trial in the AKU population, without dietary restriction and reported tyrosine levels > 500 micromol/L, both symptomatic and asymptomatic keratopathies have been observed. Perform a baseline ophthalmologic examination including slit-lamp examination prior to initiating HARLIKU treatment and regularly thereafter. Patients who develop photophobia, eye pain, or signs of inflammation such as redness, swelling, or burning of the eyes or tyrosine levels are > 500 micromol/L during treatment with HARLIKU should undergo slit-lamp re-examination and immediate measurement of the plasma tyrosine concentration.
- Painful hyperkeratotic plaques on the soles and palms.
There is no routine dietary restriction requirement for AKU patients taking HARLIKU. However, in patients who develop keratopathies, monitor plasma tyrosine levels, and implement a diet restricted in tyrosine and phenylalanine to keep the plasma tyrosine level below 500 micromol/L. Consider temporarily interrupting HARLIKU until resolution of symptoms.
5.2 Leukopenia and Severe Thrombocytopenia
In clinical trials, patients with hereditary tyrosinemia type 1 (HT-1) treated with another oral formulation of nitisinone and dietary restriction developed reversible leukopenia (3%), thrombocytopenia (3%), or both (1.5%). Ten percent of patients in Trial 1 developed thrombocytopenia [see Adverse Reactions (6.1)]. No patients developed infections or bleeding as a result of the episodes of leukopenia and thrombocytopenia. Monitor platelet and white blood cell counts during HARLIKU therapy.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of HARLIKU was evaluated in Trial 1, a three-year, open-label, randomized, no-treatment controlled trial in 40 patients with AKU. Patients were between 38 and 68 years of age (27 male, 13 female) [see Clinical Studies (14)]. Patients received either HARLIKU 2 mg orally once daily or no treatment [see Dosage and Administration (2)].
The serious adverse reactions reported with HARLIKU were ocular/visual complaints associated with elevated tyrosine levels (keratitis) [see Warnings and Precautions (5.1)]. Keratitis led to permanent treatment discontinuation in 1 (5%) treated patient.
The most common adverse reactions (>1%) reported in Trial 1 are summarized in TABLE 1.
TABLE 1. Most Common Adverse Reactions* in Patients with AKU Treated with Nitisinone**
Adverse Reactions
Nitisinone
(N=20)
n (%)
No Treatment
(N=20)
n (%)
Elevated tyrosine levels
19 (95)
0 (0)
Keratitis***
3 (15)
0 (0)
Thrombocytopenia
2 (10)
0 (0)
* reported in at least 1% of patients; ** another oral formulation of nitisinone;
*** keratitis also includes eye irritation, eye pain and photophobia. -
7 DRUG INTERACTIONS
7.1 Effects of HARLIKU on Other Drugs
Sensitive CYP2C9 Substrates
Reduce the dosage of the co-administered drug metabolized by CYP2C9 by half. Additional dosage adjustments may be needed to maintain therapeutic drug concentrations where minimal concentration changes may lead to serious adverse reactions. See prescribing information for those drugs.
Nitisinone is a moderate CYP2C9 inhibitor. Nitisinone may increase exposure of co-administered drugs metabolized by CYP2C9 [see Clinical Pharmacology (12.3)].
OAT1/OAT3 Substrates
The concomitant use of HARLIKU with OAT1/OAT3 substrates may increase the risk of adverse reactions related to the co-administered drug. See prescribing information for those drugs.
Nitisinone is an OAT1/OAT3 inhibitor which can lead to increased exposure of the co-administered drug. [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from published case reports with nitisinone use in pregnant women are insufficient to identify a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. Animal reproduction studies have been conducted for nitisinone. In these studies, nitisinone was administered to mice and rabbits during organogenesis with oral doses of nitisinone up to 600 and 240 times respectively, the maximum recommended human daily dose (MRHDD) of 2 mg/day. In mice, nitisinone caused incomplete skeletal ossification of fetal bones and decreased pup survival at doses 12 times the MRHDD, and increased gestational length at doses 120 times the MRHDD. In rabbits, nitisinone caused maternal toxicity and incomplete skeletal ossification of fetal bones at doses 48 times the MRHDD (see Data).The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal DataIn an embryo-fetal development study in mice, nitisinone was administered via oral gavage to pregnant mice at dose levels of 12, 120 and 600 times the maximum recommended human daily dose (MRHDD) of 2 mg/day. Nitisinone caused incomplete skeletal ossification of fetal bones and decreased pup survival at doses 12 times the MRHDD and increased gestational length at doses 120 times the MRHDD.
In an embryo-fetal development study in rabbits, nitisinone was administered via oral gavage to pregnant rabbits at dose levels of 48, 120 and 240 times the MRHDD of 2 mg/day. Nitisinone caused maternal toxicity and incomplete skeletal ossification of fetal bones at doses 48 times the MRHDD.
In a single dose-group study in rats given 100 mg/kg (486 times the MRHDD of 2 mg/day), reduced litter size, decreased pup weight at birth, and decreased survival of pups after birth was demonstrated.
8.2 Lactation
Risk Summary
There are no data on the presence of nitisinone in human milk, the effects on the breastfed infant, or the effects on milk production. Data suggest that nitisinone is present in rat milk due to findings of ocular toxicity and lower body weight seen in drug naive nursing rat pups. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for HARLIKU and any potential adverse effects on the breastfed infant from HARLIKU or from the underlying maternal condition.8.4 Pediatric Use
The safety and effectiveness of HARLIKU have not been established in pediatric patients with AKU.
8.5 Geriatric Use
There was 1 patient 65 years of age and older in the clinical studies for AKU [see Clinical Studies (14)]. Of the total number of HARLIKU-treated patients in these studies, 1 (2.5 %) were 65 years of age and older, while 0 (0%) were 75 years of age and older.
Clinical studies of HARLIKU did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients.
-
11 DESCRIPTION
HARLIKU (nitisinone) is a hydroxyphenyl-pyruvate dioxygenase inhibitor.
Nitisinone occurs as a white to yellowish-white, crystalline powder. It is practically insoluble in water, soluble in 2M sodium hydroxide and in methanol, and sparingly soluble in alcohol.
The chemical name of nitisinone is 2-(2-nitro-4-trifluoromethylbenzoyl) cyclohexane-1,3-dione. The molecular formula is C14H10F3NO5 and the molecular weight is 329.23. The structural formula is:
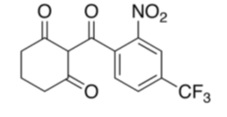
Each HARLIKU (nitisinone) tablet contains 2 mg of nitisinone to be administered orally. The inactive ingredients are glyceryl dibehenate and lactose monohydrate.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Nitisinone is a competitive inhibitor of hydroxyphenyl-pyruvate dioxygenase, an enzyme upstream of homogentisate 1,2-dioxygenase (HGD) in the tyrosine catabolic pathway.
12.2 Pharmacodynamics
In patients with AKU, HGA accumulates in various tissues and urine. In an open-label, single center, randomized, no-treatment controlled trial nitisinone treatment resulted in reduction of urinary HGA concentrations in patients with AKU [see Clinical Studies (14)].
Nitisinone exposure-response relationship and time course of pharmacodynamic response for the effectiveness have not been fully characterized.
Nitisinone inhibits catabolism of the amino acid tyrosine and can result in elevated plasma levels of tyrosine in patients with AKU. Treatment with nitisinone does not require routine dietary restriction in patients with AKU; however, patients who develop keratopathies should be monitored and dietary restriction of tyrosine and phenylalanine should be implemented [see Warnings and Precautions (5.1)].
12.3 Pharmacokinetics
The single-dose pharmacokinetics of nitisinone tablets have been studied in healthy adult subjects. Nitisinone pharmacokinetic parameters are presented as geometric mean [range] unless otherwise specified. Nitisinone maximum concentration (Cmax) and area under the curve from time 0 to 120 hours (AUC0-120h) were 1278 [780 to 1649] ng/mL and 77874 [42335 to 104211] ngh/mL following oral administration of 10 mg (5 times the recommended dosage) nitisinone under fasting conditions.
Absorption
Nitisinone time to Cmax (Tmax) is 3.5 hours (median, ranging from 1 to 4 hours) following single oral administration of 10 mg (5 times the recommended dosage) nitisinone under fasting conditions.
Effect of Food: In a food effect study, a high-fat and high-calorie breakfast (973.6 cal distributed in carbohydrate 250.1 cal, proteins 157 cal, fat 566.5 cal) did not significantly affect the total exposure (AUC0-120h) and Cmax of nitisinone following single oral administration of 10 mg nitisinone (5 times the recommended dosage). The median Tmax was delayed to 6 hours under fed conditions [see Dosage and Administration (2)].
Distribution
The arithmetic mean (SD) apparent volume of distribution of nitisinone is 8.2 (1.6) L in healthy subjects (n=23).
In vitro binding of nitisinone to human plasma proteins is greater than 95% at 50 micromolar concentration.
Elimination
The arithmetic mean (SD) terminal half-life of nitisinone is 59.3 (8.9) hours in healthy subjects (n=23). The mean (CV%) apparent plasma clearance in 18 healthy adults following multiple once daily doses of nitisinone 80 mg (40 times the recommended dosage) is 113 (16) mL/hr. The mean of the fraction of dose excreted renally as unchanged nitisinone in the urine (fe(%)) was 3% (n=3) following multiple oral doses of 80 mg (40 times the recommended dosage) daily in healthy subjects.
Metabolism
In vitro studies have shown that nitisinone is relatively stable in human liver microsomes with minor metabolism possibly mediated by CYP3A4 enzyme.
Excretion
The estimated mean (CV%) renal clearance of nitisinone was 0.003 L/h (25%).
Drug Interaction Studies
Clinical Studies
Nitisinone does not inhibit CYP2D6. Nitisinone is a moderate inhibitor of CYP2C9, and a weak inducer of CYP2E1 (TABLE 2). Nitisinone is an inhibitor of OAT1/3 (TABLE 2).
TABLE 2. Percent Change in AUC0-inf and Cmax for Co-administered Drugs in the Presence of Nitisinone in 18 Healthy Subjects
Co-administered Drug a
Dose of Co-administered Drug (Route of Administration)
Effect of Nitisinone on the Pharmacokinetics of Co-administered Drug b
AUC0-inf
Cmax
CYP2C9 Substrate Tolbutamide c
500 mg (oral)
131%↑
16%↑
CYP2E1 Substrate
Chlorzoxazone
250 mg (oral)
27%↓
18%↓
OAT1/3 Substrate Furosemide
20 mg (intravenous)
72%↑
12%↑
↑= Increased; ↓= Decreased
a The interacting drug was administered alone on Day 1 and together with nitisinone on Day 17.
b Multiple doses of 80 mg nitisinone (40 times the recommended dosage) were administered daily alone from Day 3 to Day 16.
c 16 subjects in Period 2 received nitisinone and tolbutamide while 18 subjects in Period 1 received nitisinone alone.In Vitro Studies
CYP450 Enzymes: Nitisinone does not inhibit CYP1A2, 2C19, or 3A4. Nitisinone does not induce CYP1A2, 2B6 or 3A4/5.
Transporter Systems: Nitisinone does not inhibit P-gp, BCRP, OATP1B1, OATP1B3 and OCT2. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of nitisinone was assessed in a 26-week oral (gavage) carcinogenicity study in Tg.rasH2 mice. There were no drug-related neoplastic findings in male or female Tg.rasH2 mice at doses up to 100 mg/kg/day nitisinone (approximately 243 times the maximum recommended human daily dose of 2 mg/day).
Nitisinone was not genotoxic in the Ames test and the in vivo mouse liver unscheduled DNA synthesis (UDS) test. Nitisinone was mutagenic in the mouse lymphoma cell (L5178Y/TK+/-) forward mutation test and in an in vivo mouse bone marrow micronucleus test.
-
14 CLINICAL STUDIES
The effectiveness of HARLIKU was evaluated in an open-label, single center, randomized, no-treatment controlled trial in 40 adult patients diagnosed with AKU (NCT00107783). Patients received either HARLIKU at 2 mg orally once daily or no treatment for three years.
One patient in the HARLIKU group died after experiencing atrial fibrillation and had discontinued treatment one month prior to death, and 2 patients in the no treatment control group discontinued the study early. Among the 40 patients enrolled in the trial, 27 (67.5%) were male and 13 (32.5%) were female, ranging in age from 38 to 68 with a mean age of 51.7 years. Thirty-seven (92.5%) patients were White, 2 (5%) were Asian, and 1 (2.5%) was American Indian or Alaska Native. Thirty-nine (97.5%) patients were not Hispanic or Latino and 1 (2.5%) patient was Hispanic or Latino.
HARLIKU was effective at reducing levels of urinary HGA. The HARLIKU group had an average percent reduction from baseline of 88% (95% CI: 79, 97%) after 1 year of treatment, which was sustained through three years of treatment with an average percent reduction from baseline of 91% at year 3 (95% CI: 85, 97%). In contrast, the untreated controls had an average increase of 107% from baseline to year 1 (95% CI: 0, 216%) and 108% from baseline to year 3 (95% CI: 19, 198%).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
HARLIKU (nitisinone) tablet is white to beige, round, flat which may display light yellow to brown speckles, debossed with the “strength” in mg on one side and “L” on the other side. Each tablet contains 2 mg nitisinone.
HARLIKU is supplied in a high-density polyethylene (HDPE) square bottle with a child-resistant tamper-evident polypropylene (PP) screw cap. Each bottle contains 60 tablets.
2 mg tablets: NDC: 70709-112-60
Storage and Handling
Store HARLIKU tablets at room temperature between 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
Pharmacist: Dispense in tight and light resistant container as defined in USP.
-
17 PATIENT COUNSELING INFORMATION
Ocular Symptoms and Hyperkeratotic Plaques Due to Elevated Plasma Tyrosine Levels
Advise the patient or caregiver to report any unexplained ocular or other symptoms promptly to their healthcare provider [see Warnings and Precautions (5.1)].
How Supplied/Storage and Handling
Advise the patient or caregiver to store HARLIKU in the container that it is dispensed in and keep the container tightly closed [see How Supplied/Storage and Handling (16)].
-
SPL UNCLASSIFIED SECTION
Manufactured by:
PCI Pharma Services
23-24 Tafarnaubach Industrial Estate
Tredegar, Gwent
NP22 3AA, United KingdomRivopharm SA
Centro Insema
6928 Manno, SwitzerlandMarketed by:
Cycle Pharmaceuticals Ltd
The Broers Building
21 JJ Thomson Ave
Cambridge, CB3 0FA, United KingdomPatented: see www.cyclepharma.com/product-patent-information

-
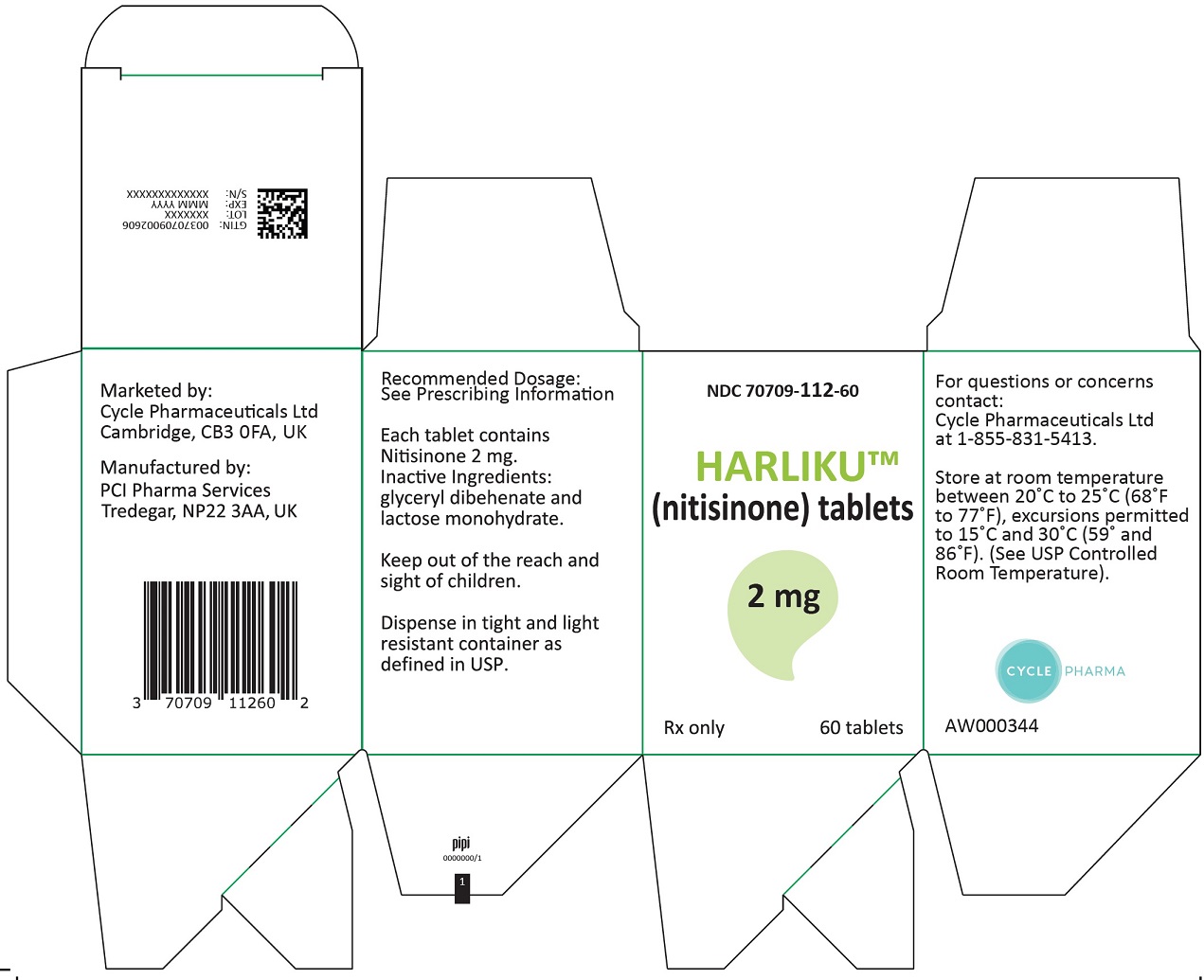
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 2 mg Carton Label
NDC: 70709-112-60
HARLIKUTM
(nitisinone) tablets2 mg
Rx only 60 tablets
-
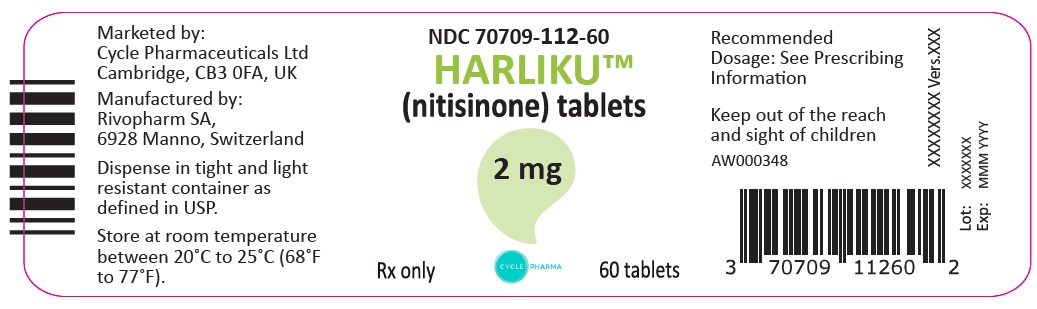
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 2 mg Bottle Label
NDC: 70709-112-60
HARLIKUTM
(nitisinone) tablets2 mg
Rx only CYCLE PHARMA 60 tablets
-
INGREDIENTS AND APPEARANCE
HARLIKU
nitisinone tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70709-112 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength nitisinone (UNII: K5BN214699) (nitisinone - UNII:K5BN214699) nitisinone 2 mg Inactive Ingredients Ingredient Name Strength GLYCERYL DIBEHENATE (UNII: R8WTH25YS2) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) Product Characteristics Color white (white to beige which may display light yellow to brown speckles) Score no score Shape ROUND (round, flat) Size 7mm Flavor Imprint Code L;2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70709-112-60 1 in 1 CARTON 07/21/2025 1 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209449 07/21/2025 Labeler - Cycle Pharmaceuticals Ltd. (218215530) Establishment Name Address ID/FEI Business Operations Rivopharm SA 480641935 MANUFACTURE(70709-112) , analysis(70709-112) , pack(70709-112) Establishment Name Address ID/FEI Business Operations Penn Pharmaceutical Services Limited 226277259 analysis(70709-112) , manufacture(70709-112) , pack(70709-112) Establishment Name Address ID/FEI Business Operations Catalent Nottingham Limited 520311192 analysis(70709-112) Establishment Name Address ID/FEI Business Operations Pharmaron Manufacturing Services (UK) Limited 739350903 api manufacture(70709-112) Establishment Name Address ID/FEI Business Operations Central Pharma Contract Packing Limited 348669123 pack(70709-112)
Trademark Results [HARLIKU]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HARLIKU 98760928 not registered Live/Pending |
Cycle Pharmaceuticals Ltd 2024-09-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.