10 PERSON ANSI- benzalkonium chloride, lidocaine, bacitracin zinc, neomycin sulfate, polymyxin b sulfate, water, benzocaine, alcohol, ibuprofen, acetaminophen, aspirin kit
10 Person ANSI by
Drug Labeling and Warnings
10 Person ANSI by is a Otc medication manufactured, distributed, or labeled by Genuine First Aid LLC, GFA Production ( Xiamen) Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- DO NOT USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- STOP USE
- DO NOT USE
- WHEN USING
- INACTIVE INGREDIENT
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- DO NOT USE
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- WHEN USING
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- STORAGE AND HANDLING
- WARNINGS
- DOSAGE & ADMINISTRATION
- PURPOSE
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- STORAGE AND HANDLING
- DO NOT USE
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
Warnings
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include: shock, facial swelling, asthma (wheezing) rash, skin reddening, blisters, hives If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains a nonsteroidal anti-inflammatory drug (NSAID), which may cause severe stomach
bleeding. The chance is higher if you: are age 60 or older, have had stomach ulcers or bleeding problems, take a blood thinner (anticoagulant) or steroid drug, take other drugs containing NSAIDs (aspirin, ibuprofen, naproxen, or others), have 3 or more alcoholic drinks every day while using this product, take more or for a longer time than directed - DO NOT USE
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- WHEN USING
-
STOP USE
Stop use and ask a doctor If:
you experience any of the following signs of stomach bleeding; feel faint; vomit blood; have bloody or black stools; have stomach pain that does get better; pain gets worse or lasts more than 10 days; fever gets worse or lasts more than 3 days; redness or swelling is present in the painful area; any new symptoms appear - PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions:
do not use more than directed; the smallest effective dose should be used; do not take longer than 10 days, unless directed by a doctor.
Adults and Children (12 years and older): Take 1 tablet every 4 to 6 hours while symptoms persist. If pain or fever does not respond to 1 tablet, 2 tablets may be used. Do not exceed 6 tablets in 24 hours, unless directed by a doctor.
Children under 12 years: Do not give to children under 12 years of age.
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
Warnings:
Liver warning: This product contains acetaminophen.
Severe liver damage may occur if: adult takes more than 12 tablets in 24 hours, which is the maximum daily amount; child takes more than 5 doses in 24 hours; taken with other drugs containing acetaminophen; adult has 3 or more alcoholic drinks every day while using this product - DO NOT USE
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
Adults and Children Take 2 tablets every 4 to 6 hours as
12 years of age needed. Do not take more than 12 tablets
or older in 24 hours.
Children 6-11 years Take 1 tablet every 4 to 6 hours as
of age needed. Do not take more than 5
tablets in 24 hours.
Children under 6 Do not use this regular strength product.
years of age This will provide more than the
recommended dose (overdose) and could
cause serious health problems. - STORAGE AND HANDLING
- GENERAL PRECAUTIONS
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox of flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include: hives, skin reddening, facial swelling, rash, asthma (wheezing), blisters, shock, If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
are age 60 or older; have had stomach ulcers or bleeding problems; take a blood thinner (anticoagulant) or steroid drug; take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others); have 3 or more alcoholic drinks every day while using this product; take more or for a longer time than directed - DO NOT USE
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- WHEN USING
-
STOP USE
Stop use and ask a doctor if
you experience any of the following signs of stomach bleeding:
feel faint; vomit blood; have bloody or black stools; have stomach
pain that does not get better; pain gets worse or lasts more than 10 days; fever gets worse or lasts more than 3 days; you have difficulty swallowing; if ringing in the ears or loss of hearing occurs; redness or swelling is present in the painful areas; any new symptoms appear - PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- WHEN USING
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
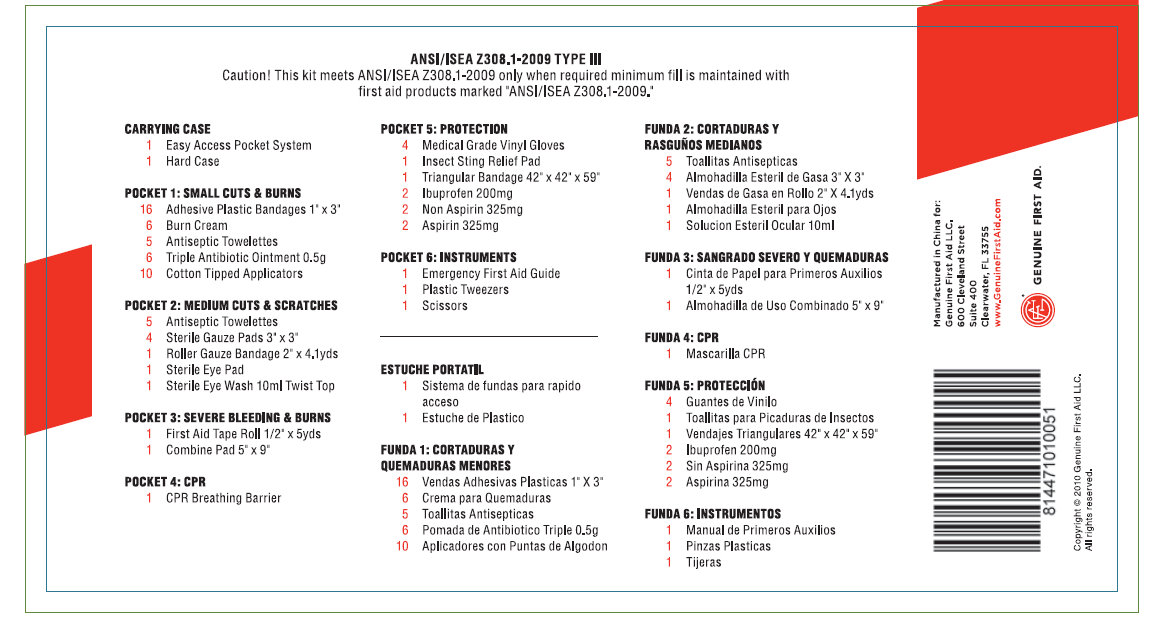
PRINCIPAL DISPLAY PANEL
ANSI/ISEA Z308.1-2009 TYPE III
Caution! This Kit meets ANSI/ISEA Z308.1-2009 only when required minimum fill is maintained with first aid products marked "ANSI/ISEA Z308.1-2009."
CARRYING CASE
1 Easy Access Pocket
System
1 Hard CasePOCKET1: SMALL CUTS AND BURNS
16 Adhesive Plastic Bandages 1"x3"
1 Burn Cream
5 Antiseptic Towelettes
6 Triple Antibiotic Ointment 0.5gr
10 Cotton Tipped Applicators
POCKET 2: MEDIUM CUTS AND SCRATCHES
5 Antiseptic Towelettes
4 Sterile Gauze Pad 3"x3"
1 Roller Gauze Bandage 2"X4.1yds
1 Sterile Eye Pads
1 Sterile Eye Wash 10ml Twist Top
POCKET 3: SEVERE BLEEDING AND BURNS
1 First Aid Tape Roll 1/2"x5 yds.
1 Combine Pad 5"X9"POCKET 4: CPR
1 CPR Breathing Barrier
POCKET 5: PROTECTION
2 Medical Grade Vinyl Gloves
1 Insect Sting Relief Pads
1 Triangular Bandage 42"x42"x59"
2 Ibuprofen 200mg
2Non Aspirin 325mg
2 Aspirin 325mg
POCKET 6: INSTRUMENTS
1 Emergency First Aid Guide
1 Plastic Tweezers
1 Scissors
Manufactured in China for:
Genuine First Aid LLC.
600 Cleveland Street
Suite 400
Clearwater FL 33755
www.GenuineFirstAid.comGENUINE FIRST AID
Copyright c 2010 Genuine First Aid LLC. All rights reserved.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
10 PERSON ANSI
benzalkonium chloride, lidocaine, bacitracin zinc, neomycin sulfate, polymyxin b sulfate, water, benzocaine, alcohol, ibuprofen, acetaminophen, aspirin kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 52124-0111 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52124-0111-1 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 6 PACKAGE 5.4 g Part 2 10 PACKAGE 8 mL Part 3 6 TUBE 3 g Part 4 1 BOTTLE 10 mL Part 5 1 PACKAGE 0.5 mL Part 6 1 PACKAGE 2 Part 7 1 PACKAGE 2 Part 8 1 PACKAGE 2 Part 1 of 8 GENUINE FIRST AID BURN ANTISEPTIC PAIN RELIEF WITH ALOE
benzalkonium chloride, lidocaine creamProduct Information Item Code (Source) NDC: 52124-0004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 0.5 in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52124-0004-1 0.9 g in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part345 04/24/2010 Part 2 of 8 ANTISEPTIC TOWELETTE
benzalkonium chloride liquidProduct Information Item Code (Source) NDC: 52124-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.40 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52124-0001-1 0.8 mL in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333 04/24/2010 Part 3 of 8 GENUINE TRIPLE ANTIBIOTIC
bacitracin zinc,neomycin sulfate,polymyxin b sulfate ointmentProduct Information Item Code (Source) NDC: 52124-0003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN ZINC 400 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN SULFATE 5 mg in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B SULFATE 5000 [iU] in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52124-0003-1 0.5 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333 04/24/2010 Part 4 of 8 STERILE ISOTONIC BUFFERED GENUINE EYEWASH
water liquidProduct Information Item Code (Source) NDC: 52124-0005 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 98.6 mL in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) EDETATE DISODIUM (UNII: 7FLD91C86K) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52124-0005-1 10 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 04/24/2010 Part 5 of 8 INSECT STING RELIEF PAD
benzocaine,alcohol liquidProduct Information Item Code (Source) NDC: 52124-0008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 6 mL in 100 mL ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 60 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52124-0008-1 0.5 mL in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part348 04/24/2010 Part 6 of 8 IBUPROFEN
ibuprofen tabletProduct Information Item Code (Source) NDC: 52124-0009 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg Inactive Ingredients Ingredient Name Strength POWDERED CELLULOSE (UNII: SMD1X3XO9M) STARCH, CORN (UNII: O8232NY3SJ) HYPROMELLOSE (UNII: 3NXW29V3WO) LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color white (White) Score no score Shape ROUND Size 10mm Flavor Imprint Code 44;352 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52124-0009-1 2 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075010 04/24/2010 Part 7 of 8 NON-ASPIRIN
acetaminophen tabletProduct Information Item Code (Source) NDC: 52124-0010 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) STEARIC ACID (UNII: 4ELV7Z65AP) POVIDONE (UNII: FZ989GH94E) Product Characteristics Color white (WHITE) Score no score Shape ROUND Size 11mm Flavor Imprint Code AZ;234 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52124-0010-1 2 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part343 04/24/2010 Part 8 of 8 ASPIRIN
aspirin tabletProduct Information Item Code (Source) NDC: 52124-0011 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 325 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color white (White) Score no score Shape ROUND Size 11mm Flavor Imprint Code 44;157;ASPIRIN Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52124-0011-1 2 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part343 04/24/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333 04/24/2010 Labeler - Genuine First Aid LLC (619609857) Establishment Name Address ID/FEI Business Operations GFA Production ( Xiamen) Co., Ltd 421256261 manufacture
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.