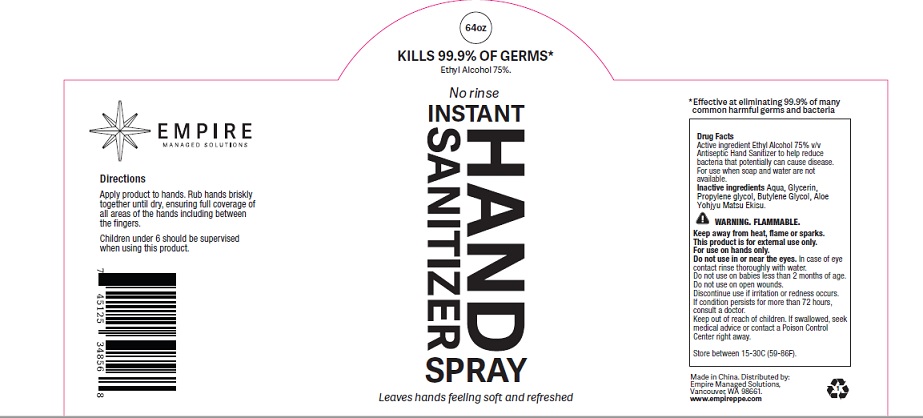
Uses
Antiseptic Hand Sanitizer to help reduce bacteria that potentially can cause disease. For use when soap and water are not available.
Warnings
FLAMMABLE.
Keep away from heat, flame or sparks.
This product is for external use only.
For use on hands only.
Do not use in or near the eyes.In case of eye contact, rinse thoroughly with water.
Do not use on babies less than 2 months of age.
Do not use on open wounds.
Discontinue use if irritation or redness occurs.
If condition persists for more than 72 hours, consult a doctor.
Keep out of reach of children. If swallowed, seek medical advice or contact a Poison Control Center right away.
Direction
Apply product to hands. Rub hands briskly together until dry, ensuring full coverage of
all areas of the hands, including between the fingers.
Children under 6 should be supervised when using this product.
Inactive ingredient
Glycerin, Water, Aloe, Propylene Glycol, Butylene Glycol
Other information
Store between 15-30C (59-86F).
Product label