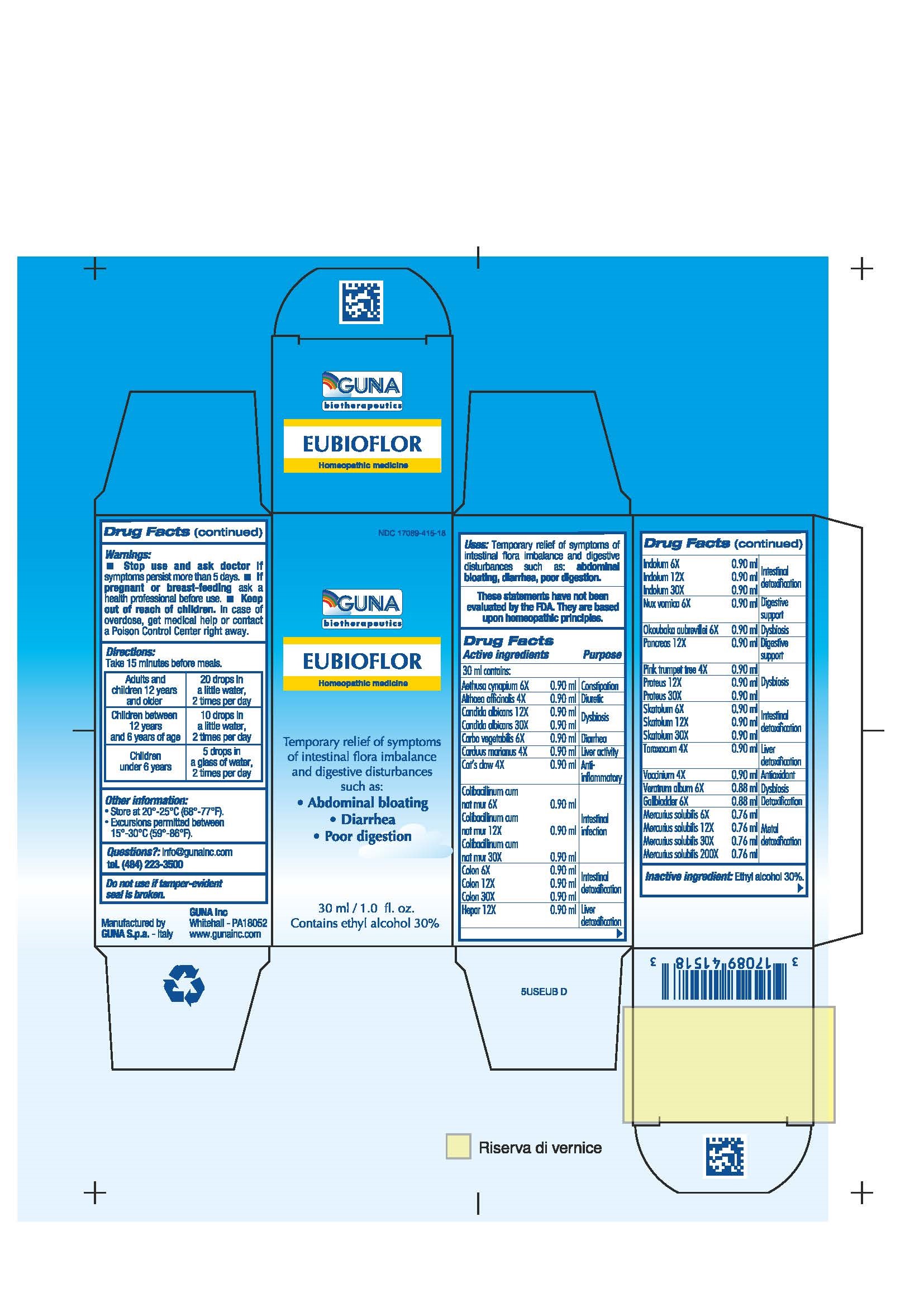
EUBIOFLOR- activated charcoal - aethusa cynapium - althaea officinalis leaf - bilberry - candida albicans - uncaria tomentosa - escherichia coli - indole - mercurius solubilis - okoubaka aubrevillei bark - pork liver - proteus vulgaris - silybum marianum seed - skatole - strychnos nux-vomica seed - sus scrofa colon - sus scrofa gall bladder - sus scrofa pancreas - tabebuia impetiginosa bark - taraxacum officinale - veratrum album root - solution/ drops
EUBIOFLOR by
Drug Labeling and Warnings
EUBIOFLOR by is a Homeopathic medication manufactured, distributed, or labeled by Guna spa. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
ACTIVE INGREDIENTS/PURPOSE
- AETHUSA CYNAPIUM 6X CONSTIPATION
- ALTHAEA OFFICINALIS 4X DIURETIC
- CANDIDA ALBICANS 12X,30X DYSBIOSIS
- CARBO VEGETALIS 6X DIARRHEA
- CARDUUS MARIANUS 4X LIVER ACTIVITY
- CAT'S CLAW 4X ANTI-INFLAMMATORY
- COLIBACILLINUM CUM NATRUM MURIATICUM 6X,12X, 30X INTESTINAL INFECTION
- COLON 6X,12X, 30X INTESTINAL DETOXIFICATION
- GALLBLADDER 6X DETOXIFICATION
- HEPAR 12X LIVER DETOXIFICATION
- INDOLUM 6X,12X, 30X INTESTINAL DETOXIFICATION
- MERCURIUS SOLUBILIS 6X,12X, 30X, 200X METAL DETOXIFICATION
- NUX VOMICA 6X DIGESTIVE SUPPORT
- OKOUBAKA AUBREVILLEI 6X DYSBIOSIS
- PANCREAS 12X DIGESTIVE SUPPORT
- PINK TRUMPET TREE 4X DYSBIOSIS
- PROTEUS 12X, 30X DYSBIOSIS
- SKATOLUM 6X,12X,30X INTESTINAL DETOXIFICATION
- TARAXACUM 4X LIVER DETOXIFICATION
- VACCINIUM 4X ANTIOXIDANT
- VERATRUM ALBUM 6X DYSBIOSIS
- USES
- WARNINGS
- PREGNANCY
- WARNINGS
- DIRECTIONS
- QUESTIONS
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EUBIOFLOR
activated charcoal - aethusa cynapium - althaea officinalis leaf - bilberry - candida albicans - uncaria tomentosa - escherichia coli - indole - mercurius solubilis - okoubaka aubrevillei bark - pork liver - proteus vulgaris - silybum marianum seed - skatole - strychnos nux-vomica seed - sus scrofa colon - sus scrofa gall bladder - sus scrofa pancreas - tabebuia impetiginosa bark - taraxacum officinale - veratrum album root - solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 17089-415 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OKOUBAKA AUBREVILLEI BARK (UNII: MK2074187Z) (OKOUBAKA AUBREVILLEI BARK - UNII:MK2074187Z) OKOUBAKA AUBREVILLEI BARK 6 [hp_X] in 30 mL SUS SCROFA PANCREAS (UNII: 9Y3J3362RY) (SUS SCROFA PANCREAS - UNII:9Y3J3362RY) SUS SCROFA PANCREAS 12 [hp_X] in 30 mL TABEBUIA IMPETIGINOSA BARK (UNII: 6GLA1946WX) (TABEBUIA IMPETIGINOSA BARK - UNII:6GLA1946WX) TABEBUIA IMPETIGINOSA BARK 4 [hp_X] in 30 mL PROTEUS VULGARIS (UNII: 11T9HCO30O) (PROTEUS VULGARIS - UNII:11T9HCO30O) PROTEUS VULGARIS 12 [hp_X] in 30 mL SKATOLE (UNII: 9W945B5H7R) (SKATOLE - UNII:9W945B5H7R) SKATOLE 30 [hp_X] in 30 mL TARAXACUM OFFICINALE (UNII: 39981FM375) (TARAXACUM OFFICINALE - UNII:39981FM375) TARAXACUM OFFICINALE 4 [hp_X] in 30 mL BILBERRY (UNII: 9P2U39H18W) (BILBERRY - UNII:9P2U39H18W) BILBERRY 4 [hp_X] in 30 mL VERATRUM ALBUM ROOT (UNII: QNS6W5US1Z) (VERATRUM ALBUM ROOT - UNII:QNS6W5US1Z) VERATRUM ALBUM ROOT 6 [hp_X] in 30 mL MERCURIUS SOLUBILIS (UNII: 324Y4038G2) (MERCURIUS SOLUBILIS - UNII:324Y4038G2) MERCURIUS SOLUBILIS 200 [hp_X] in 30 mL AETHUSA CYNAPIUM (UNII: M6936L953C) (AETHUSA CYNAPIUM - UNII:M6936L953C) AETHUSA CYNAPIUM 6 [hp_X] in 30 mL ALTHAEA OFFICINALIS LEAF (UNII: E2QQV92338) (ALTHAEA OFFICINALIS LEAF - UNII:E2QQV92338) ALTHAEA OFFICINALIS LEAF 4 [hp_X] in 30 mL CANDIDA ALBICANS (UNII: 4D7G21HDBC) (CANDIDA ALBICANS - UNII:4D7G21HDBC) CANDIDA ALBICANS 30 [hp_X] in 30 mL ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 6 [hp_X] in 30 mL SILYBUM MARIANUM SEED (UNII: U946SH95EE) (SILYBUM MARIANUM SEED - UNII:U946SH95EE) SILYBUM MARIANUM SEED 4 [hp_X] in 30 mL CAT'S CLAW (UNII: 9060PRM18Q) (CAT'S CLAW - UNII:9060PRM18Q) CAT'S CLAW 4 [hp_X] in 30 mL ESCHERICHIA COLI (UNII: 514B9K0L10) (ESCHERICHIA COLI - UNII:514B9K0L10) ESCHERICHIA COLI 30 [hp_X] in 30 mL SUS SCROFA COLON (UNII: 94J255A0UC) (SUS SCROFA COLON - UNII:94J255A0UC) SUS SCROFA COLON 30 [hp_X] in 30 mL SUS SCROFA GALLBLADDER (UNII: B6A98VOI9I) (SUS SCROFA GALLBLADDER - UNII:B6A98VOI9I) SUS SCROFA GALLBLADDER 6 [hp_X] in 30 mL PORK LIVER (UNII: 6EC706HI7F) (PORK LIVER - UNII:6EC706HI7F) PORK LIVER 12 [hp_X] in 30 mL INDOLE (UNII: 8724FJW4M5) (INDOLE - UNII:8724FJW4M5) INDOLE 12 [hp_X] in 30 mL STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 6 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 9 mL in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17089-415-18 1 in 1 BOX 12/21/2018 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/27/2010 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-415)
Trademark Results [EUBIOFLOR]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EUBIOFLOR 78825278 3187338 Live/Registered |
GUNA Inc. 2006-02-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.