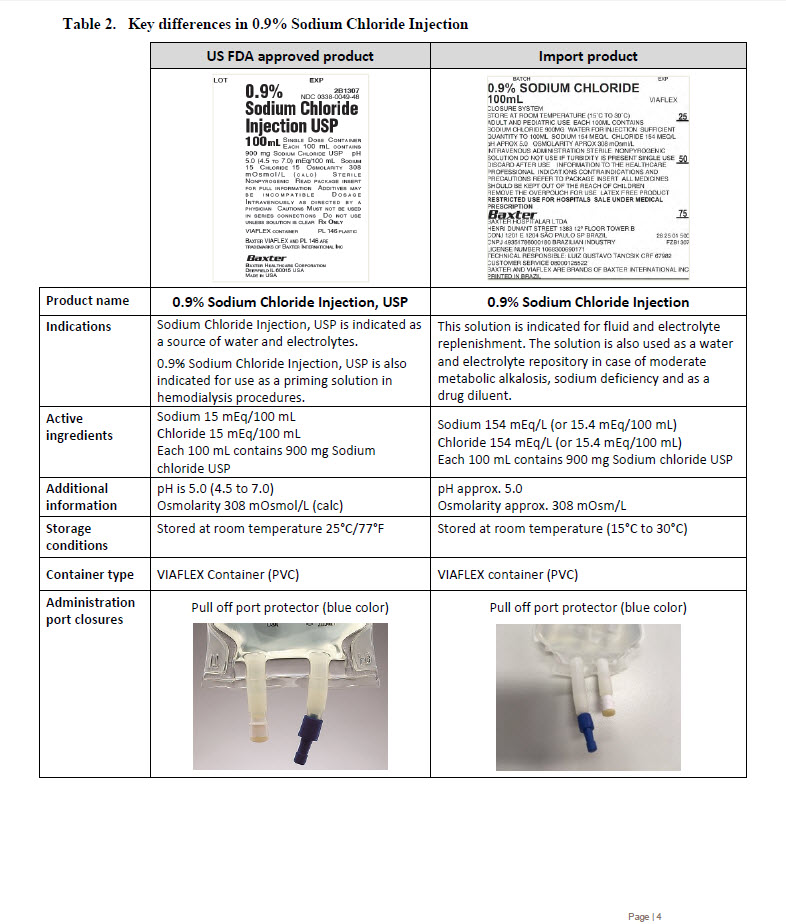
0.9% Sodium Chloride
Health Care Provider Letter
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
BATCH
EXP
0.9% Sodium Chloride
100mL
VIAFLEX
CLOSURE SYSTEM
STORE AT ROOM TEMPERATURE (15°C TO 30°C)
ADULT AND PEDIATRIC USE EACH 100ML CONTAINS
SODIUM CHLORIDE 900MG WATER FOR INJECTION SUFFICIENT
QUANTITY TO 100ML SODIUM 154 MEQ/L CHLORIDE 154 MEQ/L
pH APPROX 5.0 OSMOLARITY APPROX 308 mOsml/L
INTRAVENOUS ADMINISTRATION STERILE NONPYROGENIC
SOLUTION DO NOT USE IF TURBIDITY IS PRESENT SINGLE USE
DISCARD AFTER USE INFORMATION TO THE HEALTHCARE
PROFESSIONAL INDICATIONS CONTRAINDICATIONS AND
PRECAUTIONS REFER TO PACKAGE INSERT ALL MEDICINES
SHOULD BE KEPT OUT OF THE REACH OF CHILDREN
REMOVE THE OVERPOUCH FOR USE LATEX FREE PRODUCT
RESTRICTED USE FOR HOSPITALS SALE UNDER MEDICAL
PRESCRIPTION
BAXTER
BAXTER HOSPITALAR LTDA
HENRI DUNANT STREET 1383 12º FLOOR TOWER B
CONJ 1201 E 1204 SÃO PAULO SP BRAZIL
CNPJ 49351786000180 BRAZILIAN INDUSTRY
LICENSE NUMBER 1068300690171
TECHNICAL RESPONSIBLE: LUIZ GUSTAVO TANCSIK CRF 67982
CUSTOMER SERVICE 08000125522
BAXTER AND VIAFLEX ARE BRANDS OF BAXTER INTERNATIONAL INC
PRINTED IN BRAZIL
25
50
75
28 25 01 500
FZB1307
SODIUM CHLORIDE
sodium chloride injection |
| Product Information |
| Product Type | HUMAN PRESCRIPTION DRUG | Item Code (Source) | NDC: 0338-9517 |
| Route of Administration | INTRAVENOUS |
|
| Active Ingredient/Active Moiety |
| Ingredient Name | Basis of Strength | Strength |
| SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) | SODIUM CHLORIDE | 900 mg in 100 mL |
|
| Inactive Ingredients |
| Ingredient Name | Strength |
| WATER (UNII: 059QF0KO0R) | |
|
|
| Packaging |
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
| 1 | NDC: 0338-9517-72 | 72 in 1 CARTON | 01/15/2018 | 08/01/2019 |
| 1 | | 100 mL in 1 BAG; Type 0: Not a Combination Product | | |
|
|
| Marketing Information |
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved drug for use in drug shortage | | 01/15/2018 | 08/01/2019 |
|
| Labeler - Baxter Healthcare Corporation
(005083209)
|
Baxter Healthcare Corporation