LIDOCAINE HYDROCHLORIDE AND DEXTROSE- lidocaine hydrochloride anhydrous and dextrose monohydrate injection, solution
Lidocaine Hydrochloride and Dextrose by
Drug Labeling and Warnings
Lidocaine Hydrochloride and Dextrose by is a Prescription medication manufactured, distributed, or labeled by B. Braun Medical Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Lidocaine Hydrochloride and 5% Dextrose Injection USP is a sterile, nonpyrogenic solution prepared from lidocaine hydrochloride and dextrose in water for injection.
Lidocaine hydrochloride is designated chemically as 2-(Diethylamino)-2',6'-acetoxylidide monohydrochloride. The solution serves as a cardiac antiarrhythmic agent intended for intravenous use.
Composition - Each 100 mL contains: Solution Lidocaine
Hydrochloride
Anhydrous
USPHydrous
Dextrose
USPpH Calculated
Osmolarity
mOsmol/liter0.4% Lidocaine HCl and
5% Dextrose Injection USP0.4 g 5 g 4.4 (3.0–7.0) 280 0.8% Lidocaine HCl and
5% Dextrose Injection USP0.8 g 5 g 4.2 (3.0–7.0) 305 Water for Injection USP qs
The formulas of the active ingredients are:
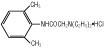
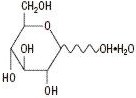
Lidocaine Hydrochloride Anhydrous USP
(M.W. 270.80)Hydrous Dextrose USP
(M.W. 198.17)Not made with natural rubber latex, PVC or DEHP.
The plastic container is made from a multilayered film specifically developed for parenteral drugs. It contains no plasticizers and exhibits virtually no leachables. The solution contact layer is a rubberized copolymer of ethylene and propylene. The container is nontoxic and biologically inert. The container-solution unit is a closed system and is not dependent upon entry of external air during administration. The container is overwrapped to provide protection from the physical environment and to provide an additional moisture barrier when necessary.
The closure system has two ports; the one for the administration set has a tamper evident plastic protector. Refer to the Directions for Use of the container.
-
CLINICAL PHARMACOLOGY
Lidocaine hydrochloride exerts an antiarrhythmic effect by increasing the electric stimulation threshold of the ventricle during diastole. In usual therapeutic doses, lidocaine hydrochloride produces no change in myocardial contractility, in systemic arterial pressure, or in absolute refractory period.
About 90% of an administered dose of the drug is metabolized in the liver. The remaining 10% is excreted unchanged via the kidneys.
Lidocaine toxicity is related to systemic blood levels. The decreased clearance and longer half-life of lidocaine should be taken into consideration with prolonged (24 hour) infusions. Constant rate of infusion may result in toxic accumulation of lidocaine. Infusion should be reduced to approximately one-half to compensate for decreased rate of clearance and concomitant or prior administration of propranolol may further increase blood concentrations by as much as 30% in patients without cardiac or hepatic failure. In clinical studies, patients over 65 years showed decreased lidocaine clearance. This was partly due to the tendency of elderly patients to have lower body weight and the increased risk of cardiac failure in these patients.
This solution provides approximately 170 calories per liter.
-
INDICATIONS AND USAGE
Lidocaine hydrochloride administered intravenously is specifically indicated in the acute management of (1) ventricular arrhythmias occurring during cardiac manipulations, such as cardiac surgery and (2) life-threatening arrhythmias which are ventricular in origin, such as occur during acute myocardial infarction.
-
CONTRAINDICATIONS
Lidocaine hydrochloride is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type.
Lidocaine should not be used in patients with Stokes-Adams syndrome, Wolff-Parkinson-White syndrome, or with severe degrees of sinoatrial, atrioventricular, or intraventricular block.
Solutions containing dextrose may be contraindicated in patients with known allergy to corn or corn products.
-
WARNINGS
Constant monitoring with an electrocardiograph is essential to the proper administration of lidocaine hydrochloride intravenously. Signs of excessive depression of cardiac conductivity, such as prolongation of the PR interval, widening of the QRS interval or the appearance or aggravation of arrhythmias, should be followed by prompt cessation of the intravenous infusion of this agent. It is mandatory to have emergency resuscitative equipment and drugs immediately available to manage adverse reactions involving cardiovascular, respiratory, or central nervous systems. Occasional acceleration of ventricular rate may occur when lidocaine hydrochloride is administered to patients with atrial fibrillation. Evidence for proper usage in pediatric patients is limited. Anaphylactic reactions may occur following administration of lidocaine hydrochloride. In the case of severe reaction, discontinue the use of the drug.
Because dosages of this drug are titrated to response (see DOSAGE AND ADMINISTRATION), no additives should be made to Lidocaine Hydrochloride and 5% Dextrose Injection USP.
-
PRECAUTIONS
General
Caution should be employed in the repeated use of lidocaine hydrochloride in patients with severe liver or renal disease because accumulation may occur and lead to toxic phenomena, since lidocaine hydrochloride is metabolized mainly in the liver and excreted by the kidneys. The drug should also be used with caution in patients with hypovolemia and shock, and in all forms of heart block (see CONTRAINDICATIONS and WARNINGS).
In patients with sinus bradycardia or incomplete heart block, the administration of lidocaine hydrochloride intravenously for the elimination of ventricular ectopic beats without prior acceleration in heart rate (e.g., by isoproterenol or by electric pacing) may promote more frequent and serious ventricular arrhythmias or complete heart block (see CONTRAINDICATIONS).
Most potent anesthetic agents, local anesthetics of the amide type which includes lidocaine, and muscle relaxants of both depolarizing and nondepolarizing types have been associated with malignant hyperthermia.
Care should be taken in the administration of intravenous fluids in patients with compromised myocardial function to avoid fluid overload or disturbances of serum electrolyte concentrations which might interfere with cardiac conduction or result in congestive heart failure.
Do not use plastic containers in series connection.
If administration is controlled by a pumping device, care must be taken to discontinue pumping action before the container runs dry or air embolism may result. If administration is not controlled by a pumping device, refrain from applying excessive pressure (>300mmHg) causing distortion to the container such as wringing or twisting. Such handling could result in breakage of the container.
These solutions are intended for intravenous administration using sterile equipment. It is recommended that intravenous administration apparatus be replaced at least once every 24 hours.
Use only if solution is clear and container and seals are intact.
Laboratory Tests
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations, and acid-base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation.
Drug Interactions
Lidocaine should be used with caution in patients with digitalis toxicity accompanied by atrioventricular block (see CONTRAINDICATIONS).
Coadministration of propranolol or cimetidine with lidocaine has been reported to reduce clearance from the plasma and may result in toxic accumulation of the drug (see CLINICAL PHARMACOLOGY).
When lidocaine is administered with other antiarrhythmic drugs such as phenytoin, procainamide, propranolol, amiodarone, or quinidine, the cardiac effects may be additive or antagonistic and toxic effects may be additive. Phenytoin may stimulate the hepatic metabolism of lidocaine, but the clinical significance of this effect is not known.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term animal studies have not been performed to evaluate carcinogenic potential of lidocaine; nor have studies been conducted to assess the mutagenic potential of lidocaine or its potential to affect fertility.
Pregnancy
Teratogenic Effects
Pregnancy Category B
Reproduction studies have been performed in rats at doses up to 5 times the human dose and have revealed no evidence of harm to the fetus due to lidocaine hydrochloride. There are, however, no adequate well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Lidocaine Hydrochloride and 5% Dextrose Injection USP is administered to a nursing woman.
Pediatric Use
The safety and effectiveness of lidocaine has not been established in pediatric patients (neonates to adolescents). (see WARNINGS and DOSAGE AND ADMINISTRATION.)
Geriatric Use
Lidocaine is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION).
-
ADVERSE REACTIONS
Systemic reactions of the following types have been reported:
- Central Nervous System: Light-headedness; drowsiness; dizziness; apprehension; euphoria; tinnitus; blurred or double vision; vomiting; sensation of heat, cold or numbness; twitching; tremors; convulsions; unconsciousness; respiratory depression and arrest.
- Cardiovascular System: Hypotension; cardiovascular arrest; and bradycardia which may lead to cardiac arrest.
- Hematologic Effects: methemoglobinemia
- Allergic reactions, including anaphylactic reactions, may occur but are infrequent. There have been no reports of cross-sensitivity between lidocaine hydrochloride and procainamide or between lidocaine hydrochloride and quinidine.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
Therapy of ventricular arrhythmias is often initiated with a single IV bolus of 1 mg/kg of Lidocaine Hydrochloride Injection USP. Following acute treatment by bolus in patients in whom arrhythmias tend to recur and who are incapable of receiving oral antiarrhythmic agents, intravenous infusion of Lidocaine Hydrochloride and 5% Dextrose Injection USP is administered continuously.
Rate of Administration
Adults (20 to 50 mcg/kg/min):
Average 70 kg adult mg/min mL/hr mL/min 0.4% Lidocaine Hydrochloride and
5% Dextrose Injection USP
(4 mg lidocaine hydrochloride/mL)1–4 15–60 0.25–1.0 0.8% Lidocaine Hydrochloride and
5% Dextrose Injection USP
(8 mg lidocaine hydrochloride/mL)1–4 7.5–30 0.12–0.5 Pediatric Patients (30 mcg/kg/min).1
Pharmacokinetic data indicate reduced elimination of lidocaine after prolonged infusion (24 hours) with resultant prolongation of the half-life to approximately three times that seen following a single administration. Failure to adjust the rate of infusion in keeping with this altered ability to eliminate lidocaine may result in toxic accumulation of the drug in the patient's serum.2
Intravenous infusions of lidocaine hydrochloride must be administered under constant ECG monitoring to avoid potential overdosage and toxicity. Intravenous infusion should be terminated as soon as the patient's basic cardiac rhythm appears to be stable or at the earliest signs of toxicity. It should rarely be necessary to continue intravenous infusions beyond 24 hours. As soon as possible and when indicated, patients should be changed to an oral antiarrhythmic agent for maintenance therapy.
Caution: Concentrated solutions of lidocaine hydrochloride (greater than 0.2%) should be administered by carefully calibrated infusion devices.
- 1 Standards and Guidelines for Cardiopulmonary Resuscitation (CPR) and Emergency Cardiac Care (ECC). American Heart Association, JAMA 244 (5):453–509, 1980.
- 2 LeLorier J, Grenon D, Latour Y, et al.: Pharmacokinetics of lidocaine after prolonged intravenous infusions in uncomplicated myocardial infarction. Ann Int Med 87:700–702, 1977.
Pediatric Use
Therapy should be initiated with a single IV bolus of 1 mg/kg of Lidocaine Hydrochloride Injection USP. A maintenance intravenous infusion of Lidocaine Hydrochloride and 5% Dextrose Injection USP administered at a recommended infusion rate of 30 mcg/kg/min may be given.1
Geriatric Use
Patients with reduced hepatic function or diminished hepatic blood flow (as in heart failure and after cardiac surgery), or those over 70 years of age should receive half the usual loading dose and also should be given lower maintenance levels of intravenous lidocaine. Patients over 65 years may benefit from dosing based upon body weight (see CLINICAL PHARMACOLOGY and PRECAUTIONS, Geriatric Use).
Lidocaine hydrochloride should not be added to blood transfusion assemblies.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
-
HOW SUPPLIED
Lidocaine Hydrochloride and 5% Dextrose Injection USP is supplied sterile and nonpyrogenic in Full Fill 500 mL and 250 mL EXCEL® Containers packaged 24 per case.
Fill NDC REF Solution 2 g Lidocaine Hydrochloride: 500 mL 0264-9594-10 P5941 0.4% Lidocaine Hydrochloride and 5% Dextrose Injection USP 250 mL 0264-9598-20 P5982 0.8% Lidocaine Hydrochloride and 5% Dextrose Injection USP 1 g Lidocaine Hydrochloride: 250 mL 0264-9594-20 P5942 0.4% Lidocaine Hydrochloride and 5% Dextrose Injection USP Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. Protect from freezing. It is recommended that the product be stored at room temperature (25°C); however, brief exposure up to 40°C does not adversely affect the product.
Storage in automated dispensing machines: Brief exposure up to 2 weeks to ultraviolet or fluorescent light does not adversely affect the product labeling legibility; prolonged exposure can cause fading of the red label. Rotate stock frequently.
- SPL UNCLASSIFIED SECTION
-
Directions for Use of EXCEL® Container
Do not admix with other drugs.
Caution: Do not use plastic containers in series connection.
To Open
Tear overwrap down at notch and remove solution container. Check for minute leaks by squeezing solution container firmly. If leaks are found, discard solution as sterility may be impaired.
NOTE: Before use, perform the following checks:
Inspect each container. Read the label. Ensure solution is the one ordered and is within the expiration date.
Invert container and carefully inspect the solution in good light for cloudiness, haze, or particulate matter.
Any container which is suspect should not be used.Use only if solution is clear and container and seals are intact.
Preparation for Administration
- Remove plastic protector from sterile set port at bottom of container.
- Attach administration set. Refer to complete directions accompanying set.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 500 mL Container Label
Lidocaine HCl and
5% Dextrose Injection USPREF P5941
NDC: 0264-9594-10
500 mL
EXCEL® CONTAINER
Lidocaine
2 g (4 mg/mL)
Y94-003-343
LD-261-4Each 100 mL contains: Lidocaine Hydrochloride USP 0.4 g;
Hydrous Dextrose USP 5 g; Water for Injection USP qs
pH: 4.4 (3.0-7.0); Calc. Osmolarity: 280 mOsmol/liter
WARNING: Do not admix with other drugs. Do not administer simultaneously
with blood. Sterile, nonpyrogenic. Single dose container. Do not use in series
connection. For intravenous use only. Use only if solution is clear and container
and seals are intact.
Recommended Storage: Room temperature (25°C). Avoid excessive heat. Protect
from freezing. See Package Insert.
Do not remove overwrap until ready for use. After removing the overwrap, check for minute leaks by squeezing container firmly. If leaks are found, discard solution as sterility may be impaired.
Not made with natural rubber latex, PVC or DEHP.
Rx only

EXCEL is a registered trademark of B. Braun Medical Inc.
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
Y94-003-293
LD-121-4EXP
LOT
-
PRINCIPAL DISPLAY PANEL - 250 mL Container Label
Lidocaine HCl and
5% Dextrose Injection USPREF P5942
NDC: 0264-9594-20
250 mL
EXCEL® CONTAINER
Lidocaine
1 g (4 mg/mL)
Y94-003-298
LD-270-3Each 100 mL contains: Lidocaine Hydrochloride USP 0.4 g;
Hydrous Dextrose USP 5 g; Water for Injection USP qs
pH: 4.4 (3.0-7.0)
Calc. Osmolarity: 280 mOsmol/liter
WARNING: Do not admix with other drugs. Do not administer
simultaneously with blood. Sterile, nonpyrogenic. Single dose
container. Do not use in series connection. For intravenous use
only. Use only if solution is clear and container and seals are
intact.
Recommended Storage: Room temperature (25°C). Avoid excessive
heat. Protect from freezing. See Package Insert.Do not remove overwrap until ready for use. After removing the overwrap,
check for minute leaks by squeezing container firmly. If leaks are found,
discard solution as sterility may be impaired.Not made with natural rubber latex, PVC or DEHP.
EXCEL is a registered trademark of B. Braun Medical Inc.
Rx only

B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862Y94-003-297
LD-119-4EXP
LOT
-
PRINCIPAL DISPLAY PANEL - 250 mL Container Label
Lidocaine HCl and
5% Dextrose Injection USPREF P5982
NDC: 0264-9598-20
250 mL
EXCEL® CONTAINER
Lidocaine
2 g (8 mg/mL)
Y94-003-296
LD-343-2Each 100 mL contains: Lidocaine Hydrochloride USP 0.8 g;
Hydrous Dextrose USP 5 g; Water for Injection USP qs
pH: 4.2 (3.0-7.0)
Calc. Osmolarity: 305 mOsmol/liter
Warning: Do not admix with other drugs. Do not administer
simultaneously with blood. Sterile, nonpyrogenic. Single dose
container. Do not use in series connection. For intravenous use
only. Use only if solution is clear and container and seals are
intact.
Recommended Storage: Room temperature (25°C). Avoid excessive
heat. Protect from freezing. See Package Insert.Do not remove overwrap until ready for use. After removing the overwrap,
check for minute leaks by squeezing container firmly. If leaks are found,
discard solution as sterility may be impaired.Not made with natural rubber latex, PVC or DEHP.
EXCEL is a registered trademark of B. Braun Medical Inc.
Rx only

B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862Y94-003-295
LD-120-4EXP
LOT
-
INGREDIENTS AND APPEARANCE
LIDOCAINE HYDROCHLORIDE AND DEXTROSE
lidocaine hydrochloride anhydrous and dextrose monohydrate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0264-9598 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE ANHYDROUS (UNII: EC2CNF7XFP) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 0.8 g in 100 mL DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0264-9598-20 24 in 1 CASE 04/08/1992 1 250 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019830 04/08/1992 LIDOCAINE HYDROCHLORIDE AND DEXTROSE
lidocaine hydrochloride anhydrous and dextrose monohydrate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0264-9594 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE ANHYDROUS (UNII: EC2CNF7XFP) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 0.4 g in 100 mL DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0264-9594-20 24 in 1 CASE 04/08/1992 1 250 mL in 1 CONTAINER; Type 0: Not a Combination Product 2 NDC: 0264-9594-10 24 in 1 CASE 04/08/1992 2 500 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019830 04/08/1992 Labeler - B. Braun Medical Inc. (002397347)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.