Diva Protect Antibacterial Hand Cleanser by Diva International Inc.
Diva Protect Antibacterial Hand Cleanser by
Drug Labeling and Warnings
Diva Protect Antibacterial Hand Cleanser by is a Otc medication manufactured, distributed, or labeled by Diva International Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
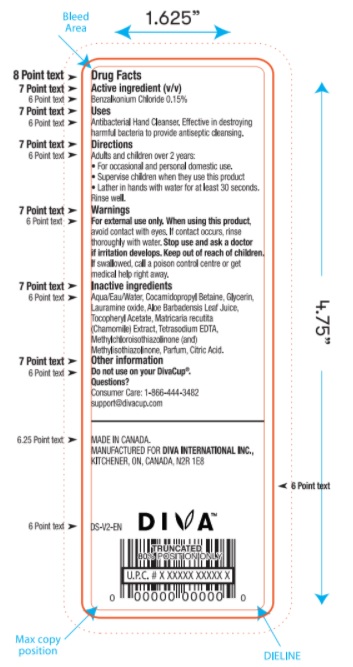
DIVA PROTECT ANTIBACTERIAL HAND CLEANSER- benzalkonium chloride liquid
Diva International Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Uses
Antibacterial Hand Cleanser. Effective in destroying harmful bacteria to provide antiseptic cleansing.
Warnings
- For external use only.
- When using this product avoid contact with eyes. If contact occurs, rinse thoroughly with water.
- Stop use and ask a doctor if irritation develops.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Adults and children over 2 years:
- For occasional and personal domestic use.
- Supervise children when they use this product
- Lather in hands with water for at least 30 seconds rinse well.
Inactive ingredients
Water, Cocamidopropyl Betaine, Glycerin, Lauramine oxide, Aloe Barbadensis Leaf Juice, Tocopheryl Acetate, Matricaria recutita (Chamomile) Extract, Tetrasodium EDTA, Methylchloroisothiazolinone (and) Methylisothiazolinone, Parfum, Citric Acid
| DIVA PROTECT ANTIBACTERIAL HAND CLEANSER
benzalkonium chloride liquid |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Diva International Inc. (207651758) |