VALGANCICLOVIR- valganciclovir hydrochloride tablet, film coated
Valganciclovir by
Drug Labeling and Warnings
Valganciclovir by is a Prescription medication manufactured, distributed, or labeled by AvKARE. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
VALGANCICLOVIR TABLETS, USP
These highlights do not include all the information needed to use VALGANCICLOVIR TABLETS safely and effectively. See full prescribing information for VALGANCICLOVIR TABLETS.
VALGANCICLOVIR tablets, for oral use
Initial U.S. Approval: 2001WARNING: HEMATOLOGIC TOXICITY, IMPAIRMENT OF FERTILITY, FETAL TOXICITY, MUTAGENESIS AND CARCINOGENESIS
See full prescribing information for complete boxed warning.
- Hematologic Toxicity: Severe leukopenia, neutropenia, anemia, thrombocytopenia, pancytopenia, bone marrow aplasia and aplastic anemia have been reported in patients treated with valganciclovir ( 5.1).
- Impairment of Fertility: Based on animal data, valganciclovir may cause temporary or permanent inhibition of spermatogenesis ( 5.2).
- Fetal Toxicity: Based on animal data, valganciclovir has the potential to cause birth defects in humans ( 5.3).
-
Mutagenesis and Carcinogenesis: Based on animal data, valganciclovir has the potential to cause cancers in humans (
5.4).
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Valganciclovir Tablets, USP are a cytomegalovirus (CMV) nucleoside analogue DNA polymerase inhibitor indicated for:
Adult Patients ( 1.1)
- Treatment of CMV retinitis in patients with acquired immunodeficiency syndrome (AIDS).
- Prevention of CMV disease in kidney, heart, and kidney-pancreas transplant patients at high risk.
Pediatric Patients ( 1.2)
- Prevention of CMV disease in heart transplant patients at high risk.
DOSAGE AND ADMINISTRATION
Adult Dosage ( 2.2) Treatment of CMV retinitis Induction: 900 mg (two 450 mg tablets) twice a day for 21 days
Maintenance: 900 mg (two 450 mg tablets) once a dayPrevention of CMV disease in heart or kidney-pancreas transplant patients 900 mg (two 450 mg tablets) once a day within 10 days of transplantation until 100 days post-transplantation Prevention of CMV disease in kidney transplant patients 900 mg (two 450 mg tablets) once a day within 10 days of transplantation until 200 days post-transplantation Pediatric Dosage ( 2.3) Prevention of CMV disease in heart transplant patients 4 months to 16 years of age Dose once a day within 10 days of transplantation until 100 days post-transplantation according to dosage algorithm (note the calculation of creatinine clearance using a modified Schwartz formula in children) - Valganciclovir tablets should be taken with food ( 2.1, 12.3).
- Valganciclovir tablets should not be broken or crushed ( 2.6).
- Adult patients should use valganciclovir tablets, not valganciclovir for oral solution ( 2.1).
- Adults with renal impairment: Adjust dose based on creatinine clearance. For adult patients receiving hemodialysis a dose recommendation cannot be given ( 2.5, 8.6, 12.3).
DOSAGE FORMS AND STRENGTHS
- Tablets: 450 mg ( 3)
CONTRAINDICATIONS
Hypersensitivity to valganciclovir or ganciclovir ( 4)
WARNINGS AND PRECAUTIONS
- Hematologic toxicity: Severe leukopenia, neutropenia, anemia, thrombocytopenia, pancytopenia, bone marrow depression, and aplastic anemia have occurred with the use of valganciclovir or ganciclovir. Avoid valganciclovir use if absolute neutrophil count is less than 500 cells/µL, platelet count is less than 25,000/µL, or hemoglobin is less than 8 g/dL. Use with caution in pre-existing cytopenias and when receiving myelosuppressive drugs or irradiation. Monitor with frequent testing of platelet and complete blood counts ( 5.1).
- Impairment of fertility: Based on animal studies, valganciclovir may cause temporary or permanent inhibition of spermatogenesis ( 5.2).
- Fetal toxicity: Based on animal studies, valganciclovir may cause fetal harm. Females of reproductive potential should use effective contraception during and following treatment and males should practice barrier contraception during and following treatment ( 5.3).
- Mutagenicity and carcinogenicity: Based on animal studies, valganciclovir is potentially mutagenic and carcinogenic ( 5.4).
- Acute renal failure: Acute renal failure may occur in elderly patients (with or without reduced renal function), patients who receive concomitant nephrotoxic drugs, or inadequately hydrated patients. Use with caution in elderly patients or those taking nephrotoxic drugs, reduce dosage in patients with renal impairment, and monitor renal function ( 2.5, 5.5, 8.5, 8.6, 12.3).
ADVERSE REACTIONS
- Adult patients: Most common adverse events and laboratory abnormalities (reported in at least one indication by greater than or equal to 20% of patients) are diarrhea, pyrexia, nausea, tremor, neutropenia, anemia, graft rejection, thrombocytopenia, and vomiting ( 6.1).
- Pediatric patients: Most common adverse events and laboratory abnormalities (reported in greater than or equal to 20% of pediatric solid organ transplant recipients) are diarrhea, pyrexia, hypertension, upper respiratory tract infection, urinary tract infection, vomiting, neutropenia, leukopenia, and headache ( 6.1).
To report SUSPECTED ADVERSE REACTIONS, contact AvKARE, Inc. at 1-855-361-3993 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
- Zidovudine: Potential to cause neutropenia and anemia. Monitor with frequent tests of white blood cell counts with differential and hemoglobin levels ( 7).
- Probenecid: May increase ganciclovir levels. Monitor for evidence of ganciclovir toxicity ( 7).
- Mycophenolate mofetil (MMF): May increase ganciclovir concentrations and levels of MMF metabolites in patients with renal impairment. Monitor for ganciclovir and MMF toxicity ( 7).
- Didanosine: May increase didanosine concentrations. Monitor for didanosine toxicity ( 7).
USE IN SPECIFIC POPULATIONS
- Lactation: Breastfeeding is not recommended with use of valganciclovir ( 8.2).
Pediatric use information for pediatric kidney transplant patients ages 4 months to 16 years and for pediatric heart transplant patients ages 1 to less than 4 months is approved for Roche Palo Alto LLC’s VALCYTE (valganciclovir hydrochloride) tablets and oral solution. However, due to Roche Palo Alto LLC’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2016
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: HEMATOLOGIC TOXICITY, IMPAIRMENT OF FERTILITY, FETAL TOXICITY, MUTAGENESIS AND CARCINOGENESIS
1 INDICATIONS AND USAGE
1.1 Adult Patients
1.2 Pediatric Patients
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
2.2 Recommended Dosage in Adult Patients with Normal Renal Function
2.3 Recommended Dosage in Pediatric Patients
2.5 Dosage Recommendation for Adult Patients with Renal Impairment
2.6 Handling and Disposal
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hematologic Toxicity
5.2 Impairment of Fertility
5.3 Fetal Toxicity
5.4 Mutagenesis and Carcinogenesis
5.5 Acute Renal Failure
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Adult Patients
14.2 Pediatric Patients
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: HEMATOLOGIC TOXICITY, IMPAIRMENT OF FERTILITY, FETAL TOXICITY, MUTAGENESIS AND CARCINOGENESIS
- Hematologic Toxicity: Severe leukopenia, neutropenia, anemia, thrombocytopenia, pancytopenia, bone marrow aplasia and aplastic anemia have been reported in patients treated with valganciclovir [see Warnings and Precautions (5.1)] .
- Impairment of Fertility: Based on animal data, valganciclovir may cause temporary or permanent inhibition of spermatogenesis [see Warnings and Precautions (5.2)] .
- Fetal Toxicity: Based on animal data, valganciclovir has the potential to cause birth defects in humans [see Warnings and Precautions (5.3)] .
- Mutagenesis and Carcinogenesis: Based on animal data, valganciclovir has the potential to cause cancers in humans [see Warnings and Precautions (5.4)] .
-
1 INDICATIONS AND USAGE
1.1 Adult Patients
Treatment of Cytomegalovirus (CMV) Retinitis: Valganciclovir Tablets, USP are indicated for the treatment of CMV retinitis in patients with acquired immunodeficiency syndrome (AIDS) [see Clinical Studies (14.1)] .
Prevention of CMV Disease: Valganciclovir Tablets, USP are indicated for the prevention of CMV disease in kidney, heart, and kidney-pancreas transplant patients at high risk (Donor CMV seropositive/Recipient CMV seronegative [D+/R-]) [see Clinical Studies (14.1)] .
1.2 Pediatric Patients
Prevention of CMV Disease: Valganciclovir Tablets, USP are indicated for the prevention of CMV disease in heart transplant patients (4 months to 16 years of age) at high risk [see Clinical Studies (14.2)] .
Pediatric use information for pediatric kidney transplant patients ages 4 months to 16 years and for pediatric heart transplant patients ages 1 to less than 4 months is approved for Roche Palo Alto LLC’s VALCYTE (valganciclovir hydrochloride) tablets and oral solution. However, due to Roche Palo Alto LLC’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
-
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
- Adult patients should use valganciclovir tablets, not valganciclovir for oral solution.
- Valganciclovir tablets should be taken with food [see Clinical Pharmacology (12.3)] .
2.2 Recommended Dosage in Adult Patients with Normal Renal Function
For dosage recommendations in adult patients with renal impairment [see Dosage and Administration (2.5)] .
Treatment of CMV Retinitis:
- Induction: The recommended dosage is 900 mg (two 450 mg tablets) taken orally twice a day for 21 days.
- Maintenance: Following induction treatment, or in adult patients with inactive CMV retinitis, the recommended dosage is 900 mg (two 450 mg tablets) taken orally once a day.
Prevention of CMV Disease:
- For adult patients who have received a heart or kidney-pancreas transplant, the recommended dosage is 900 mg (two 450 mg tablets) taken orally once a day starting within 10 days of transplantation until 100 days post-transplantation.
- For adult patients who have received a kidney transplant, the recommended dosage is 900 mg (two 450 mg tablets) taken orally once a day starting within 10 days of transplantation until 200 days post-transplantation.
2.3 Recommended Dosage in Pediatric Patients
Prevention of CMV Disease in Pediatric Heart Transplant Patients: For pediatric heart transplant patients 4 months to 16 years of age, the recommended once daily mg dose (7x BSA x CrCL) should start within 10 days of transplantation until 100 days post-transplantation.
The recommended once daily dosage of valganciclovir tablets is based on body surface area (BSA) and creatinine clearance (CrCl) derived from a modified Schwartz formula, and is calculated using the equation below:
Pediatric Dose (mg) = 7 x BSA x CrCl (calculated using a modified Schwartz formula). If the calculated Schwartz creatinine clearance exceeds 150 mL/min/1.73m 2, then a maximum value of 150 mL/min/1.73m 2 should be used in the equation. The k values used in the modified Schwartz formula are based on pediatric patient age, as shown in Table 1.
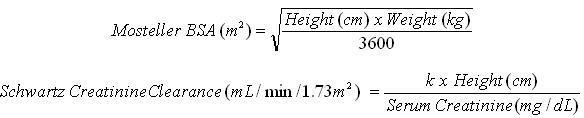
Table 1 k Values According to Pediatric Patient Age * - * The k values provided are based on the Jaffe method of measuring serum creatinine, and may require correction when enzymatic methods are used 1.
k value Pediatric Patient Age 0.33 Infants less than 1 year of age with low birth weight for gestational age 0.45 Infants less than 1 year of age with birth weight appropriate for gestational age 0.45 Children aged 1 to less than 2 years 0.55 Boys aged 2 to less than 13 years
Girls aged 2 to less than 16 years0.7 Boys aged 13 to 16 years Monitor serum creatinine levels regularly and consider changes in height and body weight and adapt the dose as appropriate during prophylaxis period.
All calculated doses should be rounded to the nearest 10 mg increment for the actual deliverable dose. If the calculated dose exceeds 900 mg, a maximum dose of 900 mg should be administered. Valganciclovir for oral solution is the preferred formulation since it provides the ability to administer a dose calculated according to the formula above; however, valganciclovir tablets may be used if the calculated doses are within 10% of available tablet strength (450 mg). For example, if the calculated dose is between 405 mg and 495 mg, one 450 mg tablet may be taken. Before prescribing valganciclovir tablets, pediatric patients should be assessed for the ability to swallow tablets.
Pediatric use information for pediatric kidney transplant patients ages 4 months to 16 years and for pediatric heart transplant patients ages 1 to less than 4 months is approved for Roche Palo Alto LLC’s VALCYTE (valganciclovir hydrochloride) tablets and oral solution. However, due to Roche Palo Alto LLC’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
2.5 Dosage Recommendation for Adult Patients with Renal Impairment
Serum creatinine levels or creatinine clearance should be monitored regularly during treatment. Dosage recommendations for adult patients with reduced renal function are provided in Table 2. For adult patients on hemodialysis (CrCl less than 10 mL/min), a dose recommendation for valganciclovir tablets cannot be given [see Use in Specific Populations (8.5, 8.6), Clinical Pharmacology (12.3)] .
Table 2 Dosage Recommendations for Adult Patients With Impaired Renal Function VALGANCICLOVIR 450 mg Tablets CrCl * (mL/min) Induction Dose Maintenance/ Prevention Dose - * An estimated creatinine clearance in adults is calculated from serum creatinine by the following formulas: (140 – age [years]) × (body weight [kg]) For males = ———————————————— (72) × (serum creatinine [mg/dL]) For females = 0.85 × male value
≥ 60 900 mg twice daily 900 mg once daily 40 – 59 450 mg twice daily 450 mg once daily 25 – 39 450 mg once daily 450 mg every 2 days 10 – 24 450 mg every 2 days 450 mg twice weekly < 10
(on hemodialysis)not recommended not recommended Dosing in pediatric patients with renal impairment can be done using the recommended equations because CrCl is a component in the calculation [see Dosage and Administration (2.3)] .
2.6 Handling and Disposal
Caution should be exercised in the handling of valganciclovir tablets. Tablets should not be broken or crushed. Because valganciclovir is considered a potential teratogen and carcinogen in humans, caution should be observed in handling broken tablets [see Warnings and Precautions (5.3, 5.4)] . Avoid direct contact with broken or crushed tablets with skin or mucous membranes. If such contact occurs, wash thoroughly with soap and water, and rinse eyes thoroughly with plain water.
Handle and dispose valganciclovir tablets according to guidelines for antineoplastic drugs because ganciclovir shares some of the properties of antitumor agents (i.e., carcinogenicity and mutagenicity) 2 .
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Valganciclovir is contraindicated in patients who have had a demonstrated clinically significant hypersensitivity reaction (e.g., anaphylaxis) to valganciclovir, ganciclovir, or any component of the formulation [see Adverse Reactions (6.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hematologic Toxicity
Severe leukopenia, neutropenia, anemia, thrombocytopenia, pancytopenia, bone marrow aplasia, and aplastic anemia have been reported in patients treated with Valganciclovir or ganciclovir. Valganciclovir should be avoided if the absolute neutrophil count is less than 500 cells/µL, the platelet count is less than 25,000/µL, or the hemoglobin is less than 8 g/dL. Valganciclovir should also be used with caution in patients with pre-existing cytopenias, or who have received or who are receiving myelosuppressive drugs or irradiation. Cytopenia may occur at any time during treatment and may worsen with continued dosing. Cell counts usually begin to recover within 3 to 7 days after discontinuing drug.
Due to the frequency of neutropenia, anemia, and thrombocytopenia in patients receiving valganciclovir [see Adverse Reactions (6.1)] , complete blood counts with differential and platelet counts should be performed frequently, especially in patients in whom ganciclovir or other nucleoside analogues have previously resulted in leukopenia, or in whom neutrophil counts are less than 1000 cells/µL at the beginning of treatment. Increased monitoring for cytopenias may be warranted if therapy with oral ganciclovir is changed to valganciclovir, because of increased plasma concentrations of ganciclovir after valganciclovir administration [see Clinical Pharmacology (12.3)] .
5.2 Impairment of Fertility
Based on animal data with ganciclovir, valganciclovir at the recommended human doses may cause temporary or permanent inhibition of spermatogenesis in males, and may cause suppression of fertility in females. Advise patients that fertility may be impaired with use of valganciclovir [see Use in Specific Populations (8.1, 8.3), Nonclinical Toxicology (13.1)] .
5.3 Fetal Toxicity
Ganciclovir may cause fetal toxicity when administered to pregnant women based on findings in animal studies. When given to pregnant rabbits at dosages resulting in 2-times the human exposure (based on AUC), ganciclovir caused malformations in multiple organs of the fetuses. Maternal and fetal toxicity were also observed in pregnant mice and rabbits. Therefore, valganciclovir has the potential to cause birth defects. Pregnancy should be avoided in female patients taking valganciclovir and in females with male partners taking valganciclovir. Females of reproductive potential should be advised to use effective contraception during treatment and for at least 30 days following treatment with valganciclovir. Similarly, males should be advised to practice barrier contraception during and for at least 90 days following treatment with valganciclovir [see Dosage and Administration (2.6), Use in Specific Populations (8.1, 8.3), Nonclinical Toxicology (13.1)].
5.4 Mutagenesis and Carcinogenesis
Animal data indicate that ganciclovir is mutagenic and carcinogenic. Valganciclovir should therefore be considered a potential carcinogen in humans [see Dosage and Administration (2.6), Nonclinical Toxicology (13.1)] .
5.5 Acute Renal Failure
Acute renal failure may occur in:
- Elderly patients with or without reduced renal function. Caution should be exercised when administering valganciclovir to geriatric patients, and dosage reduction is recommended for those with impaired renal function [see Dosage and Administration (2.5), Use in Specific Populations (8.5, 8.6)] .
- Patients receiving potential nephrotoxic drugs. Caution should be exercised when administering valganciclovir to patients receiving potential nephrotoxic drugs.
- Patients without adequate hydration. Adequate hydration should be maintained for all patients.
-
6 ADVERSE REACTIONS
The following serious adverse events are discussed in greater detail in other sections of the labeling:
- Hematologic toxicity [see Boxed Warning, Warnings and Precautions (5.1)] .
- Acute renal failure [see Warnings and Precautions (5.5)] .
The most common adverse events and laboratory abnormalities reported in at least one indication by greater than or equal to 20% of adult patients treated with valganciclovir tablets are diarrhea, pyrexia, nausea, tremor, neutropenia, anemia, graft rejection, thrombocytopenia, and vomiting. The most common reported adverse events and laboratory abnormalities reported in greater than or equal to 20% of pediatric solid organ transplant recipients treated with valganciclovir for oral solution or tablets are diarrhea, pyrexia, hypertension, upper respiratory tract infection, urinary tract infection, vomiting, neutropenia, leukopenia, and headache.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse event rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect rates observed in practice.
Valganciclovir, a prodrug of ganciclovir, is rapidly converted to ganciclovir after oral administration. Adverse events known to be associated with ganciclovir usage can therefore be expected to occur with valganciclovir.
Adverse Events in Adults:
Treatment of CMV Retinitis in AIDS Patients: In a clinical study for the treatment of CMV retinitis in HIV-infected patients, the adverse events reported by patients receiving valganciclovir tablets (n=79) or intravenous ganciclovir (n=79) for 28 days of randomized therapy (21 days induction dose and 7 days maintenance dose), respectively, included diarrhea (16%, 10%), nausea (8%, 14%), headache (9%, 5%), and catheter-related infections (3%, 11%). The incidence of adverse events was similar between the group who received valganciclovir tablets and the group who received intravenous ganciclovir, with the exception of catheter-related infections, which occurred with greater frequency in patients randomized to receive intravenous ganciclovir. The frequencies of neutropenia (ANC less than 500/μL) were 11% for patients receiving valganciclovir tablets compared with 13% for patients receiving intravenous ganciclovir. Anemia (Hgb less than 8 g/dL) occurred in 8% of patients in each group. Other laboratory abnormalities occurred with similar frequencies in the two groups.
Adverse events and abnormal laboratory values data are available for 370 patients who received maintenance therapy with valganciclovir tablets 900 mg once daily in two open-label clinical trials. Approximately 252 (68%) of these patients received valganciclovir tablets for more than nine months (maximum duration was 36 months). Table 3 and Table 4 show the pooled adverse event data and abnormal laboratory values from these patients.
Table 3 Pooled Selected Adverse Events Reported in greater than or equal to 5% of Patients who Received Valganciclovir Tablets Maintenance Therapy for CMV Retinitis Patients with CMV Retinitis Adverse Events According to Body System Valganciclovir Tablets
(N=370)
%Gastrointestinal system Diarrhea 41 Nausea 30 Vomiting 21 Abdominal pain 15 Body as a whole Pyrexia 31 Headache 22 Central and peripheral nervous system Insomnia 16 Peripheral neuropathy 9 Paresthesia 8 Special senses Retinal detachment 15 Table 4 Pooled Laboratory Abnormalities Reported in Patients Who Received Valganciclovir Tablets Maintenance Therapy for the Treatment of CMV Retinitis Patients with CMV Retinitis Laboratory Abnormalities Valganciclovir Tablets
(N=370)
%Neutropenia: ANC/µL < 500 19 500 – < 750 17 750 – < 1000 17 Anemia: Hemoglobin g/dL < 6.5 7 6.5 – < 8.0 13 8.0 – < 9.5 16 Thrombocytopenia: Platelets/µL < 25000 4 25000 – < 50000 6 50000 – < 100000 22 Serum Creatinine: mg/dL > 2.5 3 > 1.5 – 2.5 12 Prevention of CMV Disease in Selected Solid Organ Transplantation: Table 5 shows selected adverse events regardless of severity and drug relationship with an incidence of greater than or equal to 5% from a clinical trial (up to 28 days after study treatment) where heart, kidney, kidney-pancreas and liver transplant patients received valganciclovir tablets (N=244) or oral ganciclovir (N=126) until Day 100 post-transplant. The majority of the adverse events were of mild or moderate intensity.
Table 5 Percentage of Selected Grades 1-4 Adverse Events Reported in greater than or equal to 5% of Adult Patients From a Study of Solid Organ Transplant Patients Adverse Event Valganciclovir Tablets
(N=244)
%Oral Ganciclovir
(N=126)
%Diarrhea 30 29 Tremors 28 25 Graft rejection 24 30 Nausea 23 23 Headache 22 27 Insomnia 20 16 Hypertension 18 15 Vomiting 16 14 Pyrexia 13 14 Table 6 shows selected adverse events regardless of severity and drug relationship with an incidence of greater than or equal to 5% from another clinical trial where kidney transplant patients received either valganciclovir once daily starting within 10 days post-transplant until Day 100 post-transplant followed by 100 days of placebo or valganciclovir once daily starting within 10 days post-transplant until Day 200 post-transplant. The overall safety profile of valganciclovir did not change with the extension of prophylaxis until Day 200 post-transplant in high risk kidney transplant patients.
Table 6 Percentage of Selected Grades 1-4 Adverse Events Reported in greater than or equal to 5% of Adult Patients from a Study of Kidney Transplant Patients Adverse Event Valganciclovir Tablets
Day 100 Post-transplant
(N=164)
%Valganciclovir Tablets
Day 200 Post-transplant
(N=156)
%Diarrhea 26 31 Tremors 12 17 Hypertension 13 12 Nausea 11 11 Pyrexia 12 9 Transplant rejection 9 6 Headache 10 6 Insomnia 7 6 Vomiting 3 6 Adverse events not included in Table 5 and Table 6, which either occurred at a frequency of greater than or equal to 5% in clinical studies with solid organ transplant patients, or were selected serious adverse events reported in studies with patients with CMV retinitis or in studies with solid organ transplant patients with a frequency of less than 5% are listed below.
Allergic reactions: valganciclovir hypersensitivity
Bleeding complications: potentially life-threatening bleeding associated with thrombocytopenia
Central and peripheral nervous system: paresthesia, dizziness (excluding vertigo), convulsion
Gastrointestinal disorders: abdominal pain, constipation, dyspepsia, abdominal distention, ascites
General disorders and administration site disorders: fatigue, pain, edema, peripheral edema, weakness
Hemic system: anemia, neutropenia, thrombocytopenia, pancytopenia, bone marrow depression, aplastic anemia, febrile neutropenia
Hepatobiliary disorders: abnormal hepatic function
Infections and infestations: pharyngitis/nasopharyngitis, upper respiratory tract infection, urinary tract infection, local and systemic infections and sepsis, postoperative wound infection
Injury, poisoning, and procedural complications: postoperative complications, postoperative pain, increased wound drainage, wound dehiscence
Metabolism and nutrition disorders: hyperkalemia, hypokalemia, hypomagnesemia, hyperglycemia, appetite decreased, dehydration, hypophosphatemia, hypocalcemia
Musculoskeletal and connective tissue disorders: back pain, arthralgia, muscle cramps, limb pain
Psychiatric disorders: depression, psychosis, hallucinations, confusion, agitation
Renal and urinary disorders: renal impairment, dysuria, decreased creatinine clearance
Respiratory, thoracic and mediastinal disorders: cough, dyspnea, rhinorrhea, pleural effusion
Skin and subcutaneous tissue disorders: dermatitis, pruritus, acne
Vascular disorders: hypotension
Laboratory abnormalities reported with valganciclovir tablets in two studies in solid adult organ transplant patients are listed in Table 7 and Table 8.
Table 7 Selected Laboratory Abnormalities Reported in a Study of Adult Solid Organ Transplant Patients * Laboratory Abnormalities Valganciclovir Tablets (N=244)
%Ganciclovir Capsules
(N=126)
%- * Laboratory abnormalities are those reported by investigators.
Neutropenia: ANC/µL < 500 5 3 500 – < 750 3 2 750 – < 1000 5 2 Anemia: Hemoglobin g/dL < 6.5 1 2 6.5 – < 8.0 5 7 8.0 – < 9.5 31 25 Thrombocytopenia: Platelets/µL < 25000 0 2 25000 – < 50000 1 3 50000 – < 100000 18 21 Serum Creatinine: mg/dL > 2.5 14 21 > 1.5 – 2.5 45 47 Table 8 Selected Laboratory Abnormalities Reported in a Study of Adult Kidney Transplant Patients * Laboratory Abnormalities Valganciclovir Tablets
Day 100 Post-transplant
(N=164)
%Valganciclovir Tablets
Day 200 Post-transplant
(N=156)
%- * Laboratory abnormalities are those reported by investigators.
Neutropenia: ANC/µL < 500 9 10 500 – < 750 6 6 750 – < 1000 7 5 Anemia: Hemoglobin g/dL < 6.5 0 1 6.5 – < 8.0 5 1 8.0 – < 9.5 17 15 Thrombocytopenia: Platelets/µL < 25000 0 0 25000 – < 50000 1 0 50000 – < 100000 7 3 Serum Creatinine: mg/dL > 2.5 17 14 > 1.5 – 2.5 50 48 Adverse Events in Pediatric Patients:
Valganciclovir for oral solution and tablets have been studied in 109 pediatric solid organ transplant patients who were at risk for developing CMV disease (aged 4 months to 16 years) and in 24 neonates with symptomatic congenital CMV disease (aged 8 to 34 days), with duration of ganciclovir exposure ranging from 2 to 100 days [see Use in Specific Populations (8.4), Clinical Studies (14.2)] .
Prevention of CMV Disease in Pediatric Solid Organ Transplant Patients: The most frequently reported adverse events (greater than 10% of patients), regardless of seriousness and drug relationship in pediatric solid organ transplant patients taking valganciclovir until Day 100 post-transplant were diarrhea, pyrexia, upper respiratory tract infection, hypertension, vomiting, anemia, neutropenia, constipation, nausea and transplant rejection.
In general, the safety profile was similar in pediatric patients compared to that observed in adult patients. However, the rates of certain adverse events and laboratory abnormalities, such as upper respiratory tract infection, pyrexia, nasopharyngitis, anemia, and abdominal pain were reported more frequently in pediatric patients than in adults [see Use in Specific Populations (8.4), Clinical Studies (14.2)]. Neutropenia was reported with higher incidence in the two pediatric studies as compared to adults, but there was no correlation between neutropenia and infections observed in the pediatric population.
Pediatric use information for pediatric kidney transplant patients ages 4 months to 16 years and for pediatric heart transplant patients ages 1 to less than 4 months is approved for Roche Palo Alto LLC’s VALCYTE (valganciclovir hydrochloride) tablets and oral solution. However, due to Roche Palo Alto LLC’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
6.2 Postmarketing Experience
The following adverse events have been identified during post-approval use of valganciclovir. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. As valganciclovir is rapidly and extensively converted to ganciclovir, any adverse events associated with ganciclovir might also occur with valganciclovir.
– Anaphylaxis
– Decreased fertility in males
In general, the adverse events reported during the postmarketing use of valganciclovir were similar to those identified during the clinical trials.
To report SUSPECTED ADVERSE REACTIONS contact AvKARE, Inc. at 1-855-361-3993; email drugsafety@avkare.com; or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
7 DRUG INTERACTIONS
In vivo drug-drug interaction studies were not conducted with valganciclovir. However, because valganciclovir is rapidly and extensively converted to ganciclovir, drug-drug interactions associated with ganciclovir will be expected for valganciclovir tablets. Established and other potentially significant drug interactions conducted with ganciclovir are listed in Table 9.
Table 9 Established and Other Potentially Significant Drug Interactions With Ganciclovir Name of the Concomitant Drug Change in the Concentration of Ganciclovir or Concomitant Drug Clinical Comment Zidovudine ↓ Ganciclovir
↑ ZidovudineZidovudine and valganciclovir each have the potential to cause neutropenia and anemia
Probenecid ↑ Ganciclovir Patients taking probenecid and valganciclovir should be monitored for evidence of ganciclovir toxicity
Mycophenolate Mofetil (MMF) ↔ Ganciclovir (in patients with normal renal function)
↔ MMF (in patients with normal renal function)Patients with renal impairment should be monitored carefully as levels of MMF metabolites and ganciclovir may increase
Didanosine ↓ Ganciclovir
↑ DidanosinePatients should be closely monitored for didanosine toxicity -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
After oral administration, valganciclovir (prodrug) is converted to ganciclovir (active drug) and, therefore, valganciclovir is expected to have reproductive toxicity effects similar to ganciclovir. In animal studies, ganciclovir caused maternal and fetal toxicity and embryo-fetal mortality in pregnant mice and rabbits as well as teratogenicity in rabbits at exposures two-times the human exposure. There are no available human data on use of valganciclovir or ganciclovir in pregnant women to establish the presence or absence of drug-associated risk. The background risk of major birth defects and miscarriage for the indicated populations is unknown. However, the background risk in the U.S. general population of major birth defects is 2-4% and the risk of miscarriage is 15-20% of clinically recognized pregnancies. Advise pregnant women of the potential risk to the fetus [see Warnings and Precautions (5.3), Use in Specific Populations (8.3)].
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Most maternal CMV infections are asymptomatic or they may be associated with a self-limited mononucleosis-like syndrome. However, in immunocompromised patients (i.e., transplant patients or patients with AIDS) CMV infections may be symptomatic and may result in significant maternal morbidity and mortality. The transmission of CMV to the fetus is a result of maternal viremia and transplacental infection. Perinatal infection can also occur from exposure of the neonate to CMV shedding in the genital tract. Approximately 10% of children with congenital CMV infection are symptomatic at birth. Mortality in these infants is about 10% and approximately 50-90% of symptomatic surviving newborns experience significant morbidity, including mental retardation, sensorineural hearing loss, microcephaly, seizures, and other medical problems. The risk of congenital CMV infection resulting from primary maternal CMV infection may be higher and of greater severity than that resulting from maternal reactivation of CMV infection.
Data
Animal Data
At doses resulting in two-times the human exposure of ganciclovir (all dose comparisons presented are based on the human AUC following a single intravenous infusion of 5 mg per kg of ganciclovir) resulted in maternal and embryofetal toxicity in pregnant mice and rabbits as well as teratogenicity in the rabbits. Fetal resorptions were present in at least 85% of rabbits and mice. Rabbits showed increased embryofetal mortality, growth retardation of the fetuses and structural abnormalities of multiple organs of the fetuses including the palate (cleft palate), eyes (anophthalmia/microphthalmia), brain (hydrocephalus), jaw (brachygnathia), kidneys and pancreas (aplastic organs). Increased embryofetal mortality was also seen in mice. Daily intravenous doses of approximately 1.7-times the human exposure (based on AUC) administered to female mice prior to mating, during gestation, and during lactation caused hypoplasia of the testes and seminal vesicles in the male offspring, as well as pathologic changes in the nonglandular region of the stomach.
Data from an ex-vivo human placental model showed that ganciclovir crosses the human placenta. The transfer occurred by passive diffusion and was not saturable over a concentration range of 1 to 10 mg/mL.
8.2 Lactation
Risk Summary
No data are available regarding the presence of valganciclovir (prodrug) or ganciclovir (active drug) in human milk, the effects on the breastfed infant, or the effects on milk production. The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV. Advise nursing mothers that breastfeeding is not recommended during treatment with valganciclovir because of the potential for serious adverse events in nursing infants and because of the potential for transmission of HIV [see Boxed Warning, Warnings and Precautions (5.1, 5.2, 5.3, 5.4), Nonclinical Toxicology (13.1)] .
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Females of reproductive potential should undergo pregnancy testing before initiation of valganciclovir [see Use in Specific Populations (8.1)] .
Contraception
Females
Because of the mutagenic and teratogenic potential of valganciclovir, females of reproductive potential should be advised to use effective contraception during treatment and for at least 30 days following treatment with valganciclovir [see Dosage and Administration (2.6), Warnings and Precautions (5.3, 5.4), Nonclinical Toxicology (13.1)] .
Males
Because of its mutagenic potential, males should be advised to practice barrier contraception during and for at least 90 days following, treatment with valganciclovir [see Dosage and Administration (2.6), Warnings and Precautions (5.3), Nonclinical Toxicology (13.1)] .
Infertility
Valganciclovir at the recommended doses may cause temporary or permanent female and male infertility [see Warnings and Precautions (5.2), Nonclinical Toxicology (13.1)] .
8.4 Pediatric Use
Valganciclovir tablets are indicated for the prevention of CMV disease in pediatric heart transplant patients 4 months to 16 years of age at risk for developing CMV disease [see Indications and Usage (1.2), Dosage and Administration (2.3)].
Study 1 was a safety and pharmacokinetic study in pediatric solid organ transplant patients (kidney, liver, heart, and kidney/pancreas). Valganciclovir was administered once daily within 10 days of transplantation for a maximum of 100 days post-transplantation.
The use of valganciclovir for oral solution and tablets for the prevention of CMV disease in pediatric heart transplant patients 4 months to 16 years of age is based on two studies (Study 1 described above and Study 3) and was supported by previous demonstration of efficacy in adult patients [see Clinical Pharmacology (12.3), Clinical Studies (14.2)] . Study 3 was a pharmacokinetic and safety study of valganciclovir in pediatric heart transplant patients less than 4 months of age who received a single dose of valganciclovir oral solution on each of two consecutive days. A physiologically based pharmacokinetic (PBPK) model was developed based on the available pharmacokinetic data from pediatric and adult patients to support dosing in heart transplant patients less than 1 month of age. However, due to uncertainty in model predictions for neonates, valganciclovir is not indicated for prophylaxis in this age group.
The safety and efficacy of valganciclovir tablets have not been established in children for prevention of CMV disease in pediatric liver transplant patients, in kidney transplant patients less than 4 months of age, in heart transplant patients less than 1 month of age, in pediatric AIDS patients with CMV retinitis, and in infants with congenital CMV infection.
A pharmacokinetic and pharmacodynamic evaluation of valganciclovir for oral solution was performed in 24 neonates with congenital CMV infection involving the central nervous system. All patients were treated for 6 weeks with a combination of intravenous ganciclovir 6 mg per kg twice daily or valganciclovir for oral solution at doses ranging from 14 mg per kg to 20 mg per kg twice daily. The pharmacokinetic results showed that in infants greater than 7 days to 3 months of age, a dose of 16 mg per kg twice daily of valganciclovir for oral solution provided ganciclovir systemic exposures (median AUC 0-12h = 23.6 [range 16.8 – 35.5] mcg ∙h/mL; n = 6) comparable to those obtained in infants up to 3 months of age from a 6 mg per kg dose of intravenous ganciclovir twice daily (AUC 0-12h = 25.3 [range 2.4 – 89.7] mcg ∙h/mL; n = 18) or to the ganciclovir systemic exposures obtained in adults from a 900 mg dose of valganciclovir tablets twice daily. However, the efficacy and safety of intravenous ganciclovir and of valganciclovir have not been established for the treatment of congenital CMV infection in infants and no similar disease occurs in adults; therefore, efficacy cannot be extrapolated from intravenous ganciclovir use in adults.
Pediatric use information for pediatric kidney transplant patients ages 4 months to 16 years and for pediatric heart transplant patients ages 1 to less than 4 months is approved for Roche Palo Alto LLC’s VALCYTE (valganciclovir hydrochloride) tablets and oral solution. However, due to Roche Palo Alto LLC’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
8.5 Geriatric Use
Studies of valganciclovir for oral solution or tablets have not been conducted in adults older than 65 years of age. Clinical studies of valganciclovir did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Valganciclovir is known to be substantially excreted by the kidneys, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection. In addition, renal function should be monitored and dosage adjustments should be made accordingly [see Dosage and Administration (2.5), Warnings and Precautions (5.5), Use in Specific Populations (8.6), Clinical Pharmacology (12.3)] .
8.6 Renal Impairment
Dose reduction is recommended when administering valganciclovir to patients with renal impairment
[see Dosage and Administration (2.5), Warnings and Precautions (5.5), Clinical Pharmacology (12.3)] .For adult patients on hemodialysis (CrCl less than 10 mL/min) valganciclovir tablets should not be used. Adult hemodialysis patients should use ganciclovir in accordance with the dose-reduction algorithm cited in the Cytovene ®-IV complete product information section on DOSAGE AND ADMINISTRATION: Renal Impairment [see Dosage and Administration (2.5) and Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Experience With Valganciclovir Tablets: One adult developed fatal bone marrow depression (medullary aplasia) after several days of dosing that was at least 10-fold greater than recommended for the patient's estimated degree of renal impairment.
An overdose of valganciclovir could also possibly result in increased renal toxicity [see Dosage and Administration (2.5), Use in Specific Populations (8.6)] .
Because ganciclovir is dialyzable, dialysis may be useful in reducing serum concentrations in patients who have received an overdose of valganciclovir [see Clinical Pharmacology (12.3)] . Adequate hydration should be maintained. The use of hematopoietic growth factors should be considered [see Clinical Pharmacology (12.3)] .
Experience With Intravenous Ganciclovir: Reports of overdoses with intravenous ganciclovir have been received from clinical trials and during postmarketing experience. The majority of patients experienced one or more of the following adverse events:
Hematological toxicity: pancytopenia, bone marrow depression, medullary aplasia, leukopenia, neutropenia, granulocytopenia
Hepatotoxicity: hepatitis, liver function disorder
Renal toxicity: worsening of hematuria in a patient with pre-existing renal impairment, acute renal failure, elevated creatinine
Gastrointestinal toxicity: abdominal pain, diarrhea, vomiting
Neurotoxicity: generalized tremor, convulsion
-
11 DESCRIPTION
Valganciclovir Tablets, USP contains valganciclovir hydrochloride (valganciclovir HCl), a hydrochloride salt of the L-valyl ester of ganciclovir that exists as a mixture of two diastereomers. Ganciclovir is a synthetic guanine derivative active against CMV.
Valganciclovir Tablets, USP is available as a 450 mg tablet for oral administration. Each tablet contains 496.3 mg of valganciclovir HCl (corresponding to 450 mg of valganciclovir), and the inactive ingredients colloidal silicon dioxide, crospovidone, microcrystalline cellulose, povidone K-30, and stearic acid. The film-coat applied to the tablets contains polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, and iron oxide red.
Valganciclovir HCl is a white to off-white amorphous powder with a molecular formula of C 14H 22N 6O 5HCl and a molecular weight of 390.83. The chemical name for valganciclovir HCl is L-Valine, 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]-3-hydroxypropyl ester, monohydrochloride. Valganciclovir HCl is a polar hydrophilic compound with a solubility of 70 mg/mL in water at 25°C at a pH of 7.0 and an n-octanol/water partition coefficient of 0.0095 at pH 7.0. The pKa for valganciclovir HCl is 7.6.
The chemical structure of valganciclovir HCl is:
All doses in this insert are specified in terms of valganciclovir.
-
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
Because the major elimination pathway for ganciclovir is renal, dosage reductions according to creatinine clearance are required for valganciclovir tablets [see Dosage and Administration (2.5)] .
Pharmacokinetics in Adults: The pharmacokinetics of valganciclovir and ganciclovir after administration of valganciclovir tablets have been evaluated in HIV- and CMV-seropositive patients, patients with AIDS and CMV retinitis, and in solid organ transplant patients.
The ganciclovir pharmacokinetic parameters following administration of 900 mg valganciclovir tablets and 5 mg per kg intravenous ganciclovir and 1000 mg three times daily oral ganciclovir in HIV-positive/CMV-positive patients are summarized in Table 10.
Table 10 Mean Ganciclovir Pharmacokinetic * Measures in Healthy Volunteers and HIV-positive/CMV-positive Adults at Maintenance Dosage Formulation Valganciclovir Tablets Intravenous Ganciclovir Ganciclovir Capsules - * Data were obtained from single and multiple dose studies in healthy volunteers, HIV-positive patients, and HIV-positive/CMV-positive patients with and without retinitis. Patients with CMV retinitis tended to have higher ganciclovir plasma concentrations than patients without CMV retinitis.
Dosage 900 mg once daily with food 5 mg/kg once daily 1000 mg three times daily with food AUC 0-24h (µg∙h/mL) 29.1 ± 9.7
(3 studies, n=57)26.5 ± 5.9
(4 studies, n=68)Range of means 12.3 to 19.2
(6 studies, n=94)C max (µg/mL) 5.61 ± 1.52
(3 studies, n=58)9.46 ± 2.02
(4 studies, n=68)Range of means 0.955 to 1.40
(6 studies, n=94)Absolute oral bioavailability (%) 59.4 ± 6.1
(2 studies, n=32)Not Applicable Range of means 6.22 ± 1.29 to
8.53 ± 1.53 (2 studies, n=32)Elimination half-life (hr) 4.08 ± 0.76
(4 studies, n=73)3.81 ± 0.71
(4 studies, n=69)Range of means 3.86 to 5.03
(4 studies, n=61)Renal clearance (mL/min/kg) 3.21 ± 0.75
(1 study, n=20)2.99 ± 0.67
(1 study, n=16)Range of means 2.67 to 3.98
(3 studies, n=30)The area under the plasma concentration-time curve (AUC) of ganciclovir administered as valganciclovir tablets (900 mg once daily) is comparable to the AUC of ganciclovir after administration of intravenous ganciclovir (5 mg per kg once daily). The C max of ganciclovir following valganciclovir administration is 40% lower than the C max following intravenous ganciclovir administration. During maintenance dosing, ganciclovir AUC 0-24h and C max following oral ganciclovir administration (1000 mg three times daily) are lower relative to valganciclovir and intravenous ganciclovir. The ganciclovir C min following intravenous ganciclovir and valganciclovir administration are less than the ganciclovir C min following oral ganciclovir administration. The clinical significance of the differences in ganciclovir pharmacokinetics after administration of valganciclovir tablets, ganciclovir capsules, and intravenous ganciclovir is unknown.
Figure 1 Ganciclovir Plasma Concentration Time Profiles in HIV-positive/CMV-positive Patients*
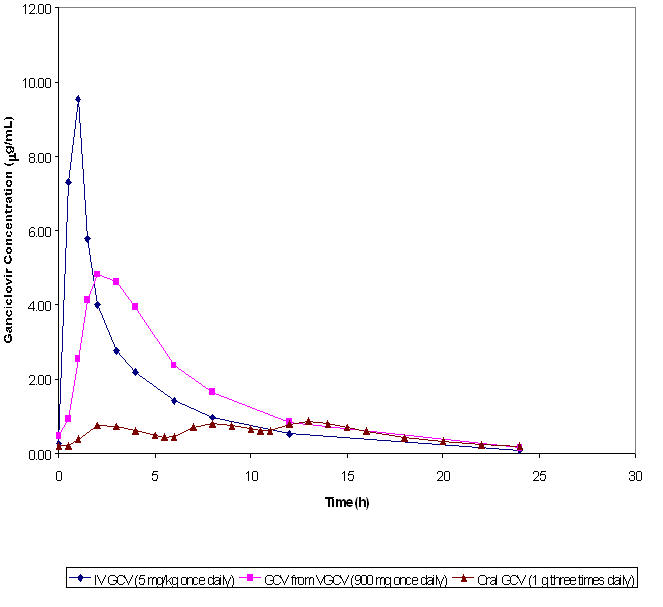
*Plasma concentration-time profiles for ganciclovir (GCV) from valganciclovir (VGCV) and intravenous ganciclovir were obtained from a multiple dose study (n=21 and n=18, respectively) in HIV-positive/CMV-positive patients with CMV retinitis. The plasma concentration-time profile for oral ganciclovir was obtained from a multiple dose study (n=24) in HIV-positive/CMV-positive patients without CMV retinitis.
In solid organ transplant recipients, the mean systemic exposure to ganciclovir was 1.7x higher following administration of 900 mg valganciclovir tablets once daily versus 1000 mg ganciclovir capsules three times daily, when both drugs were administered according to their renal function dosing algorithms. The systemic ganciclovir exposures attained were comparable across kidney, heart and liver transplant recipients based on a population pharmacokinetic evaluation (see Table 11).
Table 11 Mean Ganciclovir Pharmacokinetic Measures by Organ Transplant Type Parameter Ganciclovir Capsules Valganciclovir Tablets - * Includes kidney-pancreas
Dosage 1000 mg three times daily with food
Mean ± SD900 mg once daily with food
Mean ± SDHeart Transplant Recipients N=13
N=17
AUC 0-24h (µg ∙h/mL) 26.6 ± 11.6 40.2 ± 11.8 C max (µg/mL) 1.4 ± 0.5 4.9 ± 1.1 Elimination half-life (hr) 8.47 ± 2.84 6.58 ± 1.50 Liver Transplant Recipients N=33 N=75 AUC 0-24h (µg ∙h/mL) 24.9 ± 10.2 46.0 ± 16.1 C max (µg/mL) 1.3 ± 0.4 5.4 ± 1.5 Elimination half-life (hr) 7.68 ± 2.74 6.18 ± 1.42 Kidney Transplant Recipients * N=36 N=68 AUC 0-24h (µg ∙h/mL) 31.3 ± 10.3 48.2 ± 14.6 C max (µg/mL) 1.5 ± 0.5 5.3 ± 1.5 Elimination half-life (hr) 9.44 ± 4.37 6.77 ± 1.25 The pharmacokinetic parameters of ganciclovir following 200 days of valganciclovir administration in high-risk kidney transplant patients were similar to those previously reported in solid organ transplant patients who received valganciclovir for 100 days.
In a pharmacokinetic study in liver transplant patients, the ganciclovir AUC 0-24h achieved with 900 mg valganciclovir was 41.7 ± 9.9 mcg ∙h/mL (n=28) and the AUC 0-24h achieved with the approved dosage of 5 mg per kg intravenous ganciclovir was 48.2 ± 17.3 mcg ∙h/mL (n=27).
Absorption: Valganciclovir, a prodrug of ganciclovir, is well absorbed from the gastrointestinal tract and rapidly metabolized in the intestinal wall and liver to ganciclovir. The absolute bioavailability of ganciclovir from valganciclovir tablets following administration with food was approximately 60% (3 studies, n=18; n=16; n=28). Ganciclovir median T max following administration of 450 mg to 2625 mg valganciclovir tablets ranged from 1 to 3 hours. Dose proportionality with respect to ganciclovir AUC following administration of valganciclovir tablets was demonstrated only under fed conditions. Systemic exposure to the prodrug, valganciclovir, is transient and low, and the AUC 24 and C max values are approximately 1% and 3% of those of ganciclovir, respectively.
Food Effects: When valganciclovir tablets were administered with a high fat meal containing approximately 600 total calories (31.1 g fat, 51.6 g carbohydrates and 22.2 g protein) at a dose of 875 mg once daily to 16 HIV-positive subjects, the steady-state ganciclovir AUC increased by 30% (95% CI 12% to 51%), and the C max increased by 14% (95% CI -5% to 36%), without any prolongation in time to peak plasma concentrations (T max). Valganciclovir should be administered with food [see Dosage and Administration (2.1)] .
Distribution: Due to the rapid conversion of valganciclovir to ganciclovir, plasma protein binding of valganciclovir was not determined. Plasma protein binding of ganciclovir is 1% to 2% over concentrations of 0.5 and 51 mcg/mL. When ganciclovir was administered intravenously, the steady-state volume of distribution of ganciclovir was 0.703 ± 0.134 L/kg (n=69).
After administration of valganciclovir tablets, no correlation was observed between ganciclovir AUC and reciprocal weight; oral dosing of valganciclovir tablets according to weight is not required.
Metabolism: Valganciclovir is rapidly hydrolyzed to ganciclovir; no other metabolites have been detected. No metabolite of orally administered radiolabeled ganciclovir (1000 mg single dose) accounted for more than 1% to 2% of the radioactivity recovered in the feces or urine.
Elimination: The major route of elimination of valganciclovir is by renal excretion as ganciclovir through glomerular filtration and active tubular secretion. Systemic clearance of intravenously administered ganciclovir was 3.07 ± 0.64 mL/min/kg (n=68) while renal clearance was 2.99 ± 0.67 mL/min/kg (n=16).
The terminal half-life (t ½) of ganciclovir following oral administration of valganciclovir tablets to either healthy or HIV-positive/CMV-positive subjects was 4.08 ± 0.76 hours (n=73), and that following administration of intravenous ganciclovir was 3.81 ± 0.71 hours (n=69). In heart, kidney, kidney-pancreas, and liver transplant patients, the terminal elimination half-life of ganciclovir following oral administration of valganciclovir was 6.48 ± 1.38 hours, and following oral administration of ganciclovir capsules was 8.56 ± 3.62 hours.
Specific Populations:
Renal Impairment: The pharmacokinetics of ganciclovir from a single oral dose of 900 mg valganciclovir tablets were evaluated in 24 otherwise healthy individuals with renal impairment.
Table 12 Pharmacokinetics of Ganciclovir From a Single Oral Dose of 900 mg Valganciclovir Tablets Estimated Creatinine Clearance
(mL/min)N Apparent Clearance
(mL/min)
Mean ± SDAUC last
(µg∙h/mL)
Mean ± SDHalf-life
(hours)
Mean ± SD51-70 6 249 ± 99 49.5 ± 22.4 4.85 ± 1.4 21-50 6 136 ± 64 91.9 ± 43.9 10.2 ± 4.4 11-20 6 45 ± 11 223 ± 46 21.8 ± 5.2 ≤10 6 12.8 ± 8 366 ± 66 67.5 ± 34 Decreased renal function results in decreased clearance of ganciclovir from valganciclovir, and a corresponding increase in terminal half-life. Therefore, dosage adjustment is required for patients with impaired renal function.
Hemodialysis reduces plasma concentrations of ganciclovir by about 50% following valganciclovir administration. Adult patients receiving hemodialysis (CrCl less than 10 mL/min) cannot use valganciclovir tablets because the daily dose of valganciclovir tablets required for these patients is less than 450 mg [see Dosage and Administration (2.5) and Use in Specific Populations (8.6)] .
Pharmacokinetics in Pediatric Patients: The pharmacokinetics of ganciclovir were evaluated following the administration of valganciclovir in 63 pediatric solid organ transplant patients aged 4 months to 16 years. In these studies, patients received oral doses of valganciclovir (either valganciclovir for oral solution or tablets) to produce exposure equivalent to an adult 900 mg dose [see Dosage and Administration (2.3), Adverse Reactions (6.1), Use in Specific Populations (8.4), Clinical Studies (14.2)] .
The pharmacokinetics of ganciclovir were similar across organ types and age ranges. Based on a population pharmacokinetic evaluation, clearance is influenced by both body weight and renal function, while the central and peripheral volumes of distribution were influenced by weight [see Dosage and Administration (2.5)]. The mean total clearance was 5.3 L/hr (88.3 mL/min) for a patient with creatinine clearance of 70.4 mL/min. The mean ganciclovir C max, AUC and half-life by age and organ type in studies using the pediatric valganciclovir dosing algorithm are listed in Table 13. Relative to adult transplant patients ( Table 11), AUC values in pediatric patients were somewhat increased, but were within the range considered safe and effective in adults.
Table 13 Ganciclovir Pharmacokinetics by Age in Pediatric Solid Organ Transplant Patients a
Age Group Organ PK Parameter mean (SD) 4 months to ≤ 2 years
> 2 to < 12 years
≥ 12 years Heart
(N=12)N
AUC 0-24h
(µg ∙h/mL)
C max (µg/mL)
t 1/2 (h)6
55.4 (22.8)
8.2 (2.5)
3.8 (1.7)2
59.6 (21.0)
12.5 (1.2)
2.8 (0.9)4
60.6 (25.0)
9.5 (3.3)
4.9 (0.8)Kidney
(N=31)N
AUC 0-24h
(µg ∙h/mL)
C max (µg/mL)
t 1/2 (h)2
67.6 (13.0)
10.4 (0.4)
4.5 (1.5)10 d,e
55.9 (12.1)
8.7 (2.1)
4.8 (1.0)19
47.8 (12.4)
7.7 (2.1)
6.0 (1.3)Liver
(N=17)N
AUC 0-24h
(µg ∙h/mL)
C max (µg/mL)
t 1/2 (h)9
69.9 (37.0)
11.9 (3.7)
2.8 (1.5)6
59.4 (8.1)
9.5 (2.3)
3.8 (0.7)2
35.4 (2.8)
5.5 (1.1)
4.4 (0.2)N= number of patients
a Pharmacokinetic parameters were estimated by using population pharmacokinetic modeling.
d There was one subject in this age group who received both a kidney and liver transplant. The pharmacokinetic profile for this subject has not been included in this table as it is not possible to determine whether the effects observed are from the kidney/liver transplant or neither.
e The pharmacokinetic profiles for two subjects in this age group who received kidney transplants have not been included in this table as the data were determined to be non-evaluable.Pharmacokinetics in Geriatric Patients: The pharmacokinetic characteristics of valganciclovir in elderly patients have not been established. Because elderly individuals frequently have a reduced glomerular filtration rate, renal function should be assessed before and during administration of valganciclovir [see Dosage and Administration (2.5), Use in Specific Populations (8.5)] .
Drug Interactions: In vivo drug-drug interaction studies were not conducted with valganciclovir. However, because valganciclovir is rapidly and extensively converted to ganciclovir, interactions associated with ganciclovir will be expected for valganciclovir [see Drug Interactions (7)].
Drug-drug interaction studies were conducted in patients with normal renal function. Patients with impaired renal function may have increased concentrations of ganciclovir and the coadministered drug following concomitant administration of valganciclovir and drugs excreted by the same pathway as ganciclovir. Therefore, these patients should be closely monitored for toxicity of ganciclovir and the coadministered drug.
Table 14 and Table 15 provide a listing of established drug interaction studies with ganciclovir. Table 14 provides the effects of coadministered drug on ganciclovir plasma pharmacokinetic parameters, whereas Table 15 provides the effects of ganciclovir on plasma pharmacokinetic parameters of coadministered drug.
Table 14 Results of Drug Interaction Studies With Ganciclovir: Effects of Coadministered Drug on Ganciclovir Pharmacokinetic Parameters Coadministered Drug Ganciclovir Dosage N Ganciclovir Pharmacokinetic (PK) Parameter Zidovudine 100 mg every 4 hours 1000 mg every 8 hours 12 AUC ↓ 17 ± 25%
(range: -52% to 23%)Probenecid 500 mg every 6 hours 1000 mg every 8 hours 10 AUC ↑ 53 ± 91%
(range: -14% to 299%)
Ganciclovir renal clearance ↓ 22 ± 20%
(range: -54% to -4%)Mycophenolate Mofetil (MMF) 1.5 g single dose IV ganciclovir 5 mg/kg single dose 12 No effect on ganciclovir PK parameters observed (patients with normal renal function) Didanosine 200 mg every 12 hours administered 2 hours before ganciclovir 1000 mg every 8 hours 12 AUC ↓ 21 ± 17%
(range: -44% to 5%)
Didanosine 200 mg every 12 hours simultaneously administered with ganciclovir 1000 mg every 8 hours 12 No effect on ganciclovir PK parameters observed IV ganciclovir 5 mg/kg twice daily 11 No effect on ganciclovir PK parameters observed IV ganciclovir 5 mg/kg once daily 11 No effect on ganciclovir PK parameters observed Trimethoprim 200 mg once daily 1000 mg every 8 hours 12 Ganciclovir renal clearance ↓ 16.3%
Half-life ↑15%Table 15 Results of Drug Interaction Studies With Ganciclovir: Effects of Ganciclovir on Pharmacokinetic Parameters of Coadministered Drug Coadministered Drug Ganciclovir Dosage N Coadministered Drug Pharmacokinetic (PK) Parameter Zidovudine 100 mg every 4 hours 1000 mg every 8 hours 12 AUC 0-4 ↑ 19 ± 27%
(range: -11% to 74%)Mycophenolate Mofetil (MMF) 1.5 g single dose IV ganciclovir 5 mg/kg single dose 12 No PK interaction observed (patients with normal renal function) Didanosine 200 mg every 12 hours when administered 2 hours prior to or concurrent with ganciclovir 1000 mg every 8 hours 12 AUC 0-12 ↑ 111 ± 114%
(range: 10% to 493%)Didanosine 200 mg every 12 hours IV ganciclovir 5 mg/kg twice daily 11 AUC 0-12 ↑ 70 ± 40%
(range: 3% to 121%)C max ↑ 49 ± 48%
(range: -28% to 125%)Didanosine 200 mg every 12 hours IV ganciclovir 5 mg/kg once daily 11 AUC 0-12 ↑50 ± 26%
(range: 22% to 110%)C max ↑ 36 ± 36%
(range: -27% to 94%)Trimethoprim 200 mg once daily 1000 mg every 8 hours 12 Increase (12%) in C min Other potential drug interactions
Since ganciclovir is excreted through the kidney via glomerular filtration and active secretion [see Pharmacokinetics (12.3)] , coadministration of valganciclovir with antiretroviral drugs that share the tubular secretion pathway, such as nucleos(t)ide reverse transcriptase inhibitors, may change the plasma concentrations of valganciclovir and/or the coadministered drug.
Pediatric use information for pediatric kidney transplant patients ages 4 months to 16 years and for pediatric heart transplant patients ages 1 to less than 4 months is approved for Roche Palo Alto LLC’s VALCYTE (valganciclovir hydrochloride) tablets and oral solution. However, due to Roche Palo Alto LLC’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
12.4 Microbiology
Mechanism of Action: Valganciclovir is an L-valyl ester (prodrug) of ganciclovir that exists as a mixture of two diastereomers. After oral administration, both diastereomers are rapidly converted to ganciclovir by intestinal and hepatic esterases. Ganciclovir is a synthetic analogue of 2'-deoxyguanosine, which inhibits replication of human CMV in cell culture and in vivo.
In CMV-infected cells ganciclovir is initially phosphorylated to ganciclovir monophosphate by the viral protein kinase, pUL97. Further phosphorylation occurs by cellular kinases to produce ganciclovir triphosphate, which is then slowly metabolized intracellularly (half-life 18 hours). As the phosphorylation is largely dependent on the viral kinase, phosphorylation of ganciclovir occurs preferentially in virus-infected cells. The virustatic activity of ganciclovir is due to inhibition of the viral DNA polymerase, pUL54, synthesis by ganciclovir triphosphate.
Antiviral Activity: The quantitative relationship between the cell culture susceptibility of human herpes viruses to antivirals and clinical response to antiviral therapy has not been established, and virus sensitivity testing has not been standardized. Sensitivity test results, expressed as the concentration of drug required to inhibit the growth of virus in cell culture by 50% (EC 50), vary greatly depending upon a number of factors including the assay used. Thus, the reported EC 50 values of ganciclovir that inhibit human CMV replication in cell culture (laboratory and clinical isolates) have ranged from 0.08 to 22.94 µM (0.02 to 5.75 mcg/mL). The distribution and range in susceptibility observed in one assay evaluating 130 clinical isolates was 0 to 1 µM (35%), 1.1 to 2 µM (20%), 2.1 to 3 µM (27%), 3.1 to 4 µM (13%), 4.1 to 5 µM (5%), less than 5 µM (less than 1%). Ganciclovir inhibits mammalian cell proliferation (CC 50) in cell culture at higher concentrations ranging from 40 to greater than 1,000 µM (10.21 to greater than 250 mcg/mL). Bone marrow-derived colony-forming cells are more sensitive [CC 50 value= 2.7 to 12 µM (0.69 to 3.06 mcg/mL)].
Viral Resistance:
Cell culture: CMV isolates with reduced susceptibility to ganciclovir have been selected in cell culture. Growth of CMV strains in the presence of ganciclovir resulted in the selection of amino acid substitutions in the viral protein kinase pUL97 (M460I/V, L595S, G598D, and K599T) and the viral DNA polymerase pUL54 (D301N, N410K, F412V, P488R, L516R, C539R, L545S, F595I, V812L, P829S, L862F, D879G, and V946L).
In vivo: Viruses resistant to ganciclovir can arise after prolonged treatment or prophylaxis with valganciclovir by selection of substitutions in pUL97 and/or pUL54. Limited clinical data are available on the development of clinical resistance to ganciclovir and many pathways to resistance likely exist. In clinical isolates, seven canonical pUL97 substitutions, (M460V/I, H520Q, C592G, A594V, L595S, C603W) are the most frequently reported ganciclovir resistance-associated substitutions. These and other substitutions less frequently reported in the literature, or observed in clinical trials, are listed in Table 16.
Table 16 Summary of Resistance-associated Amino Acid Substitutions Observed in the CMV of Patients Failing Ganciclovir Treatment or Prophylaxis Note: Many additional pathways to ganciclovir resistance likely exist pUL97 L405P, A440V, M460I/V/T, V466G/M, C518Y, H520Q, del 590-593, A591D/V, C592G, A594E/G/T/V/P, L595F/S/T/W, del 595, del 595-603, E596D/G, K599E/M, del 600-601, del 597-600, del 601-603, C603W/R/S/Y, C607F/S/Y, A613V pUL54 E315D, N408D/K/S, F412C/L/S, D413A/E, L501F/I, T503I, K513E/N/R, I521T, P522A/L/S, L545S/W, Q578H/L, D588E/N, G629S, S695T, I726T/V, E756K, V781I, V787L, L802M, A809V, T813S, T821I, A834P, G841A/S, D879G, A972V, del 981-982, A987G The presence of known ganciclovir resistance-associated amino acid substitutions was evaluated in a study that extended valganciclovir CMV prophylaxis from 100 days to 200 days post-transplant in adult kidney transplant patients at high risk for CMV disease (D+/R-) [see Clinical Studies (14.1)] . Five subjects from the 100 day group and four subjects from the 200 day group meeting the resistance analysis criteria had known ganciclovir resistance-associated amino acid substitutions detected. In six subjects, the following resistance-associated amino acid substitutions were detected within pUL97: 100 day group: A440V, M460V, C592G; 200 day group: M460V, C603W. In three subjects, the following resistance-associated amino acid substitutions were detected within pUL54: 100 day group: E315D, 200 day group: E315D, P522S. Overall, the detection of known ganciclovir resistance-associated amino acid substitutions was observed more frequently in patients during prophylaxis therapy than after the completion of prophylaxis therapy (during therapy: 5/12 [42%] versus after therapy: 4/58 [7%]). The possibility of viral resistance should be considered in patients who show poor clinical response or experience persistent viral excretion during therapy.
Cross-Resistance: Cross-resistance has been reported for amino acid substitutions selected in cell culture by ganciclovir, cidofovir or foscarnet. In general, amino acid substitutions in pUL54 conferring cross-resistance to ganciclovir and cidofovir are located within the exonuclease domains and region V. Whereas, amino acid substitutions conferring cross-resistance to foscarnet are diverse, but concentrate at and between regions II (codon 696-742) and III (codon 805-845). The amino acid substitutions that resulted in reduced susceptibility to ganciclovir and either cidofovir and/or foscarnet are summarized in Table 17.
Substitutions at amino acid positions pUL97 340-400 have been found to confer resistance to ganciclovir. Resistance data based on assays that do not include this region should be interpreted cautiously.
Table 17 Summary of pUL54 Amino Acid Substitutions with Cross-Resistance between Ganciclovir, Cidofovir, and/or Foscarnet Cross-resistant to cidofovir D301N, N408D/K, N410K, F412C/L/S/V, D413E, L501I, T503I, K513E/N, L516R, I521T, P522S/A, L545S/W, Q578H, D588N, I726T/V, E756K, V812L, T813S, A834P, G841A, del 981-982, A987G Cross-resistant to foscarnet F412C, Q578H/L, D588N, E756K, V781I, V787L, L802M, A809V, V812L, T813S, T821I, A834P, G841A, del 981-982
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies have not been conducted with valganciclovir. However, upon oral administration, valganciclovir is rapidly and extensively converted to ganciclovir. Therefore, like ganciclovir, valganciclovir is a potential carcinogen.
Ganciclovir was carcinogenic in the mouse at oral doses that produced exposures approximately 0.1x and 1.4x, respectively, the mean drug exposure in humans following the recommended intravenous dose of 5 mg/kg, based on area under the plasma concentration curve (AUC) comparisons. At the higher dose there was a significant increase in the incidence of tumors of the preputial gland in males, forestomach (nonglandular mucosa) in males and females, and reproductive tissues (ovaries, uterus, mammary gland, clitoral gland and vagina) and liver in females. At the lower dose, a slightly increased incidence of tumors was noted in the preputial and harderian glands in males, forestomach in males and females, and liver in females. Ganciclovir should be considered a potential carcinogen in humans.
Valganciclovir increases mutations in mouse lymphoma cells. In the mouse micronucleus assay, valganciclovir was clastogenic. Valganciclovir was not mutagenic in the Ames Salmonella assay. Ganciclovir increased mutations in mouse lymphoma cells and DNA damage in human lymphocytes in vitro. In the mouse micronucleus assay, ganciclovir was clastogenic. Ganciclovir was not mutagenic in the Ames Salmonella assay.
Valganciclovir is converted to ganciclovir and therefore is expected to have similar reproductive toxicity effects as ganciclovir [see Warnings and Precautions (5.2)] . Ganciclovir caused decreased mating behavior, decreased fertility, and an increased incidence of embryolethality in female mice following intravenous doses that produced an exposure approximately 1.7x the mean drug exposure in humans following the dose of 5 mg per kg, based on AUC comparisons. Ganciclovir caused decreased fertility in male mice and hypospermatogenesis in mice and dogs following daily oral or intravenous administration. Systemic drug exposure (AUC) at the lowest dose showing toxicity in each species ranged from 0.03 to 0.1x the AUC of the recommended human intravenous dose. Valganciclovir caused similar effects on spermatogenesis in mice, rats, and dogs. These effects were reversible at lower doses but irreversible at higher doses. It is considered likely that ganciclovir (and valganciclovir) could cause temporary or permanent inhibition of human spermatogenesis.
-
14 CLINICAL STUDIES
14.1 Adult Patients
Induction Therapy of CMV Retinitis: In one randomized open-label controlled study, 160 patients with AIDS and newly diagnosed CMV retinitis were randomized to receive treatment with either valganciclovir tablets (900 mg twice daily for 21 days, then 900 mg once daily for 7 days) or with intravenous ganciclovir solution (5 mg per kg twice daily for 21 days, then 5 mg per kg once daily for 7 days). Study participants were: male (91%), White (53%), Hispanic (31%), and Black (11%). The median age was 39 years, the median baseline HIV-1 RNA was 4.9 log 10, and the median CD4 cell count was 23 cells/mm 3. A determination of CMV retinitis progression by the masked review of retinal photographs taken at baseline and Week 4 was the primary outcome measurement of the 3-week induction therapy. Table 18 provides the outcomes at 4 weeks.
Table 18 Week 4 Masked Review of Retinal Photographs in CMV Retinitis Study Intravenous Ganciclovir Valganciclovir Tablets Determination of CMV retinitis progression at Week 4 N=80 N=80 Progressor
Non-progressor7
637
64Death
Discontinuations due to Adverse Events
Failed to return2
1
11
2
1CMV not confirmed at baseline or no interpretable baseline photos 6 5 Maintenance Therapy of CMV Retinitis: No comparative clinical data are available on the efficacy of valganciclovir tablets for the maintenance therapy of CMV retinitis because all patients in the CMV retinitis study received open-label valganciclovir tablets after Week 4. However, the AUC for ganciclovir is similar following administration of 900 mg valganciclovir tablets once daily and 5 mg per kg intravenous ganciclovir once daily. Although the ganciclovir C max is lower following valganciclovir tablets administration compared to intravenous ganciclovir, it is higher than the C max obtained following oral ganciclovir administration [see Figure 1 in Clinical Pharmacology (12.3)] . Therefore, use of valganciclovir tablets as maintenance therapy is supported by a plasma concentration-time profile similar to that of two approved products for maintenance therapy of CMV retinitis.
Prevention of CMV Disease in Heart, Kidney, Kidney-Pancreas, or Liver Transplantation: A double blind, double-dummy active comparator study was conducted in 372 heart, liver, kidney, or kidney-pancreas transplant patients at high risk for CMV disease (D+/R-). Patients were randomized (2 valganciclovir: 1 oral ganciclovir) to receive either valganciclovir tablets (900 mg once daily) or oral ganciclovir (1000 mg three times a day) starting within 10 days of transplantation until Day 100 post-transplant. The proportion of patients who developed CMV disease, including CMV syndrome and/or tissue-invasive disease during the first 6 months post-transplant was similar between the valganciclovir tablets arm (12.1%, N=239) and the oral ganciclovir arm (15.2%, N=125). However, in liver transplant patients, the incidence of tissue-invasive CMV disease was significantly higher in the valganciclovir group compared with the ganciclovir group. These results are summarized in Table 19.
Mortality at six months was 3.7% (9/244) in the valganciclovir group and 1.6% (2/126) in the oral ganciclovir group.
Table 19 Percentage of Patients With CMV Disease, Tissue-Invasive CMV Disease or CMV syndrome by Organ Type: Endpoint Committee, 6 Month ITT Population GCV = oral ganciclovir; VGCV = valganciclovir - * Number of patients with CMV disease = Number of patients with tissue-invasive CMV disease or CMV syndrome
- † CMV syndrome was defined as evidence of CMV viremia accompanied with fever greater than or equal to 38°C on two or more occasions separated by at least 24 hours within a 7-day period and one or more of the following: malaise, leukopenia, atypical lymphocytosis, thrombocytopenia, and elevation of hepatic transaminases
CMV Disease * Tissue-Invasive CMV Disease CMV Syndrome † Organ VGCV
(N=239)GCV
(N=125)VGCV
(N=239)GCV
(N=125)VGCV
(N=239)GCV
(N=125)Liver
(n=177)19%
(22 / 118)12%
(7 / 59)14%
(16 / 118)3%
(2 / 59)5%
(6 / 118)9%
(5 / 59)Kidney
(n=120)6%
(5 / 81)23%
(9 / 39)1%
(1 / 81)5%
(2 / 39)5%
(4 / 81)18%
(7 / 39)Heart
(n=56)6%
(2 / 35)10%
(2 / 21)0%
(0 / 35)5%
(1 / 21)6%
(2 / 35)5%
(1 / 21)Kidney / Pancreas
(n=11)0%
(0 / 5)17%
(1 / 6)0%
(0 / 5)17%
(1 / 6)0%
(0 / 5)0%
(0 / 6)Prevention of CMV Disease in Kidney Transplantation: A double-blind, placebo-controlled study was conducted in 326 kidney transplant patients at high risk for CMV disease (D+/R-) to assess the efficacy and safety of extending valganciclovir CMV prophylaxis from 100 to 200 days post-transplant. Patients were randomized (1:1) to receive valganciclovir tablets (900 mg once daily) within 10 days of transplantation either until Day 200 post-transplant or until Day 100 post-transplant followed by 100 days of placebo. Extending CMV prophylaxis with valganciclovir until Day 200 post-transplant demonstrated superiority in preventing CMV disease within the first 12 months post-transplant in high risk kidney transplant patients compared to the 100 day dosing regimen (primary endpoint). These results are summarized in Table 20.
Table 20 Percentage of Kidney Transplant Patients With CMV Disease, Tissue-Invasive CMV Disease or CMV Syndrome, 12 Month ITT Population - * Number of patients with CMV disease = Number of patients with tissue-invasive CMV disease or CMV syndrome
- † CMV syndrome was defined as evidence of CMV viremia accompanied with at least one of the following: fever (greater than or equal to 38°C), severe malaise, leukopenia, atypical lymphocytosis, thrombocytopenia, and elevation of hepatic transaminases
- ‡ Two patients in the 100 day group had both tissue-invasive CMV disease and CMV syndrome; however, these patients are counted as having only tissue-invasive CMV disease.
CMV Disease* Tissue-Invasive CMV Disease CMV Syndrome† 100 Days
VGCV
(N=163)200 Days
VGCV
(N=155)100 Days
VGCV
(N=163)200 Days
VGCV
(N=155)100 Days
VGCV
(N=163)200 Days
VGCV
(N=155)Cases 36.8%
(60/163)16.8%
(26/155)1.8%
(3/163) ‡0.6%
(1/155)35.0%
(57/163)16.1%
(25/155)VGCV = valganciclovir The percentage of kidney transplant patients with CMV disease at 24 months post-transplant was 38.7% (63/163) for the 100 day dosing regimen and 21.3% (33/155) for the 200 day dosing regimen.
14.2 Pediatric Patients
Prevention of CMV in Pediatric Heart, Kidney, or Liver Transplantation: Sixty-three children, 4 months to 16 years of age, who had a solid organ transplant (kidney 33, liver 17, heart 12, and kidney/liver 1) and were at risk for developing CMV disease, were enrolled in an open-label, safety, and pharmacokinetic study of oral valganciclovir (valganciclovir for oral solution or tablets). Patients received valganciclovir once daily within 10 days after transplant until a maximum of 100 days post-transplant. The daily doses of valganciclovir were calculated at each study visit based on body surface area and a modified creatinine clearance [see Dosage and Administration (2.3)] .
The pharmacokinetics of ganciclovir were similar across organ transplant types and age ranges. The mean daily ganciclovir exposures in pediatric patients were somewhat increased relative to those observed in adult solid organ transplant patients receiving valganciclovir 900 mg once daily, but were within the range considered safe and effective in adults [see Clinical Pharmacology (12.3)] . No case of CMV syndrome or tissue-invasive CMV disease was reported within the first six months post-transplantation.
Pediatric use information for pediatric kidney transplant patients ages 4 months to 16 years and for pediatric heart transplant patients ages 1 to less than 4 months is approved for Roche Palo Alto LLC’s VALCYTE (valganciclovir hydrochloride) tablets and oral solution. However, due to Roche Palo Alto LLC’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
-
15 REFERENCES
- Brion, L.P., Fleischman, A.R., McCarton, C., Schwartz, G.J. A simple estimate of glomerular filtration rate in low birth weight infants during the first year of life: noninvasive assessment of body composition and growth. J of Ped 1986: 109(4): 698-707.
- NIOSH [2014]. NIOSH list of antineoplastic and other hazardous drugs in healthcare settings. By Connor T.H., MacKenzie B.A., DeBord D.G., Trout D.B., O’Callaghan J.P., Cincinnati, O.H.: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2014-138 (Supersedes 2012-150).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Valganciclovir Tablets, USP: Supplied as 450 mg, pink, convex oval tablets with "E114" on one side and plain on the other side. Each film-coated tablet contains 450 mg of valganciclovir as valganciclovir hydrochloride. Valganciclovir Tablets, USP, is supplied in bottles of 60 tablets (NDC: 42291-875-60).
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Serious Adverse Reactions
Inform patients that valganciclovir may cause granulocytopenia (neutropenia), anemia, thrombocytopenia and elevated creatinine levels and that dose modification or discontinuation of dosing may be required. Complete blood counts, platelet counts, and creatinine levels should be performed frequently during treatment [see Warnings and Precautions (5.1)].
Pregnancy and Contraception
Inform females of reproductive potential that valganciclovir causes birth defects in animals. Advise them to use effective contraception during and for at least 30 days following treatment with valganciclovir. Similarly, advise males to practice barrier contraception during and for at least 90 days following treatment with valganciclovir [see Use in Specific Populations (8.1, 8.3)] .
Carcinogenicity
Advise patients that valganciclovir is considered a potential carcinogen [see Nonclinical Toxicity (13.1)] .
Lactation
Advise mothers not to breast-feed if they are receiving valganciclovir because of the potential for hematologic toxicity and cancer in nursing infants, and because HIV can be passed to the baby in breast milk [see Use in Specific Populations (8.2)].
Infertility
Advise patients that valganciclovir may cause temporary or permanent female and male infertility [see Warnings and Precautions (5.2), Use in Specific Populations (8.3)] .
Impairment of Cognitive Ability
Inform patients that tasks requiring alertness may be affected including the patient’s ability to drive and operate machinery as convulsions, sedation, dizziness, ataxia and/or confusion have been reported with the use of valganciclovir [see Adverse Reactions (6.1)] .
Use in Patients with CMV Retinitis
Inform patients that valganciclovir is not a cure for CMV retinitis, and they may continue to experience progression of retinitis during or following treatment. Advise patients to have ophthalmologic follow-up examinations at a minimum of every 4 to 6 weeks while being treated with valganciclovir. Some patients will require more frequent follow-up.
Administration
Inform adult patients that they should use valganciclovir tablets, not valganciclovir for oral solution [see Dosage and Administration (2.1)] .
Inform patients to take valganciclovir with food to maximize bioavailability.
-
FDA-Approved Patient Labeling
Valganciclovir (val’gan-si’klo-vir) Tablets
What is the most important information I should know about valganciclovir?
- Valganciclovir can affect your blood cells and bone marrow causing serious and life-threatening problems. Valganciclovir can lower the amount of your white blood cells, red blood cells, and platelets. Your healthcare provider may do regular blood tests to check your blood cell counts while you are taking valganciclovir. Based on these tests, your healthcare provider may change your dose or tell you to stop taking valganciclovir.
- Valganciclovir may cause cancer. Valganciclovir causes cancer in animals. It is not known if valganciclovir causes cancer in people.
-
Valganciclovir may cause birth defects. Valganciclovir causes birth defects in animals. It is not known if valganciclovir causes birth defects in people.
If you are pregnant, talk to your healthcare provider before taking valganciclovir.
- If you are a female who can become pregnant, you should have a pregnancy test done before starting valganciclovir.
- If you are a female who can become pregnant, you should use effective birth control during treatment with valganciclovir and for at least 30 days after treatment.
- Tell your healthcare provider right away if you become pregnant while taking valganciclovir.
- Men should use a condom during treatment with valganciclovir, and for at least 90 days after treatment, if their female sexual partner can become pregnant. Talk to your healthcare provider if you have questions about birth control.
- Valganciclovir may lower the amount of sperm in a man's body and cause fertility problems. Valganciclovir may also cause fertility problems in women. Talk to your healthcare provider if this is a concern for you.
- Valganciclovir can affect your kidneys, including serious problems such as kidney failure. Your healthcare provider may do regular blood tests to check your kidney function while you are taking valganciclovir. Your healthcare provider may adjust your dose based on these tests.
- Valganciclovir changes into the medicine ganciclovir once it is in your body. Ganciclovir is also the active ingredient in Cytovene ®-IV. Do not take Cytovene ®-IV if you are taking valganciclovir. You could overdose and become very sick if valganciclovir tablets are taken with Cytovene ®-IV. Talk to your healthcare provider or pharmacist if you have questions about your medicine.
What is Valganciclovir?
Valganciclovir is a prescription antiviral medicine.
In adults, valganciclovir tablets are used:
- to treat cytomegalovirus (CMV) retinitis in people who have acquired immunodeficiency syndrome (AIDS). When CMV virus infects the eyes, it is called CMV retinitis. If CMV retinitis is left untreated, it can cause blindness.
- to prevent cytomegalovirus (CMV) disease in people who have received a heart, kidney, or kidney-pancreas transplant and who have a high risk for getting CMV disease.
In children, valganciclovir tablets or oral solution are used:
- to prevent cytomegalovirus (CMV) disease in children 4 months to 16 years of age who have received a heart transplant and have a high risk for getting CMV disease.
Pediatric use information for pediatric kidney transplant patients ages 4 months to 16 years and for pediatric heart transplant patients ages 1 to less than 4 months is approved for Roche Palo Alto LLC’s VALCYTE (valganciclovir hydrochloride) tablets and oral solution. However, due to Roche Palo Alto LLC’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
Valganciclovir does not cure CMV retinitis. You may still get retinitis or worsening of retinitis during or after treatment with valganciclovir. It is important to stay under a healthcare provider's care and have your eyes checked every 4 to 6 weeks during treatment with valganciclovir.
Who should not take valganciclovir?
Do not take valganciclovir if you are allergic to any of its ingredients or if you have ever had a serious allergic reaction to ganciclovir capsules or Cytovene-IV. Symptoms of an allergic reaction to valganciclovir may include: sudden trouble breathing, wheezing, hives all over your body, swelling around your mouth, or feeling anxious.
See the end of this leaflet for a list of the ingredients in valganciclovir tablets.
What should I tell my healthcare provider before taking valganciclovir?
Before taking valganciclovir, tell your healthcare provider if you:
- have kidney problems. Your healthcare provider may give you a lower dose of valganciclovir, or check you more often if you take valganciclovir.
- are receiving hemodialysis
- have blood cell problems
- are having radiation treatment
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if valganciclovir causes birth defects in an unborn baby.
- Tell your healthcare provider right away if you become pregnant while taking valganciclovir. See "What is the most important information I should know about valganciclovir?"
- are breastfeeding or plan to breastfeed. It is not known if valganciclovir passes into your breast milk. You should not breastfeed if you take valganciclovir.
- You should not breastfeed if you have Human Immunodeficiency Virus (HIV-1) because of the risk of passing HIV-1 to your baby.
- Talk to your healthcare provider about the best way to feed your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
Valganciclovir and other medicines may affect each other and cause serious side effects. Keep a list of your medicines to show your healthcare provider and pharmacist.
- You can ask your healthcare provider or pharmacist for a list of medicines that interact with valganciclovir.
- Do not start taking a new medicine without telling your healthcare provider. Your healthcare provider can tell you if it is safe to take valganciclovir with other medicines.
How should I take valganciclovir?
- Take valganciclovir exactly as your healthcare provider tells you. Your dose of valganciclovir will depend on your medical condition.
- Adults should only take valganciclovir tablets. Children may take either valganciclovir tablets or oral solution.
- Take valganciclovir with food.
- Do not break or crush valganciclovir tablets. Avoid contact with your skin or eyes. If you come in contact with the contents of the tablet or oral solution, wash your skin well with soap and water or rinse your eyes well with plain water.
- If you miss a dose of valganciclovir, take the missed dose as soon as you remember. Then, take the next dose at the usual scheduled time. However, if it is almost time for your next dose, do not take the missed dose.
- Do not let your valganciclovir run out. The amount of virus in your blood may increase if your medicine is stopped, even for a short time.
- If you take too much valganciclovir, call your healthcare provider or go to the nearest hospital emergency room right away.
Talk to your healthcare provider, nurse or pharmacist if you have questions about your medicine.
What should I avoid while taking valganciclovir?
- Valganciclovir can cause seizures, sleepiness, dizziness, unsteady movements, and confusion. You should not drive a car or operate other dangerous machinery until you know how valganciclovir affects you.
What are the possible side effects of valganciclovir?
Valganciclovir may cause serious side effects, including:
See " What is the most important information I should know about valganciclovir?"
Common side effects of valganciclovir in adults and children include:
- diarrhea
- nausea, vomiting
- fever
- shaky movements (tremors)
- low white cell, red cell and platelet cell counts in blood tests
- rejection of the transplanted organ (graft)
Other common side effects in children include:
- headache
- upper respiratory tract infection
- high blood pressure
- urinary tract infection
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of valganciclovir. For more information, ask your healthcare provider or pharmacist.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store valganciclovir?
- Store valganciclovir tablets at room temperature between 59°F to 86°F (15°C to 30°C).
- Do not keep valganciclovir tablets that is out of date or that you no longer need.
- Keep valganciclovir and all medicines out of the reach of children.
General information about the safe and effective use of valganciclovir
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use valganciclovir for a condition for which it was not prescribed. Do not give valganciclovir to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about valganciclovir. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about valganciclovir that is written for health professionals.
For more information about valganciclovir, call 1-855-361-3993.
What are the ingredients in valganciclovir?
Active Ingredient: valganciclovir hydrochloride
Inactive Ingredients for Tablets: colloidal silicon dioxide, crospovidone, microcrystalline cellulose, povidone K-30, and stearic acid. The film-coating applied to the tablets contains polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, and iron oxide red.
This Patient Information has been approved by the U.S. Food and Drug Administration
VALCYTE is a registered trademark of Hoffmann-La Roche Inc.
Manfactured for:
AvKARE, Inc.
Pulaski, TN 38478
Mfg. Rev. 03/16
AV 05/16 (P)
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
AvKARE
NDC 42291-875-60
VALGANCICLOVIR TABLETS, USP
CAUTION: Strict adherence to dosage recommendations is essential to avoid overdose.
DO NOT BREAK OR CRUSH TABLETS
450 mg
60 Tablets Rx Only
Attention Pharmacist:
Dispense the accompanying Patient Information to each patient.
Each film-coated tablet contains 450 mg of valganciclovir as valganciclovir hydrochloride.
USUAL DOSAGE: See package insert.Dispense in tight containers as defined in USP/NF.
Store at 25°C (77°F); excursions permitted to 15°C - 30°C (59°F - 86°F) [See USP Controlled Room Temperature].Manufactured for:
AvKARE, Inc.
Pulaski, TN 38478
Mfg. Rev. 05/14 AV 06/16 (P)N3 42291 87560 9

-
INGREDIENTS AND APPEARANCE
VALGANCICLOVIR
valganciclovir hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42291-875(NDC:0603-6330) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VALGANCICLOVIR HYDROCHLORIDE (UNII: 4P3T9QF9NZ) (GANCICLOVIR - UNII:P9G3CKZ4P5) VALGANCICLOVIR 450 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE K30 (UNII: U725QWY32X) CROSPOVIDONE (UNII: 68401960MK) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) FERRIC OXIDE RED (UNII: 1K09F3G675) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TALC (UNII: 7SEV7J4R1U) POLYVINYL ALCOHOL (UNII: 532B59J990) Product Characteristics Color pink Score no score Shape OVAL (Convex oval) Size 17mm Flavor Imprint Code E114 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42291-875-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 06/21/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA200790 06/21/2016 Labeler - AvKARE, Inc. (796560394)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.