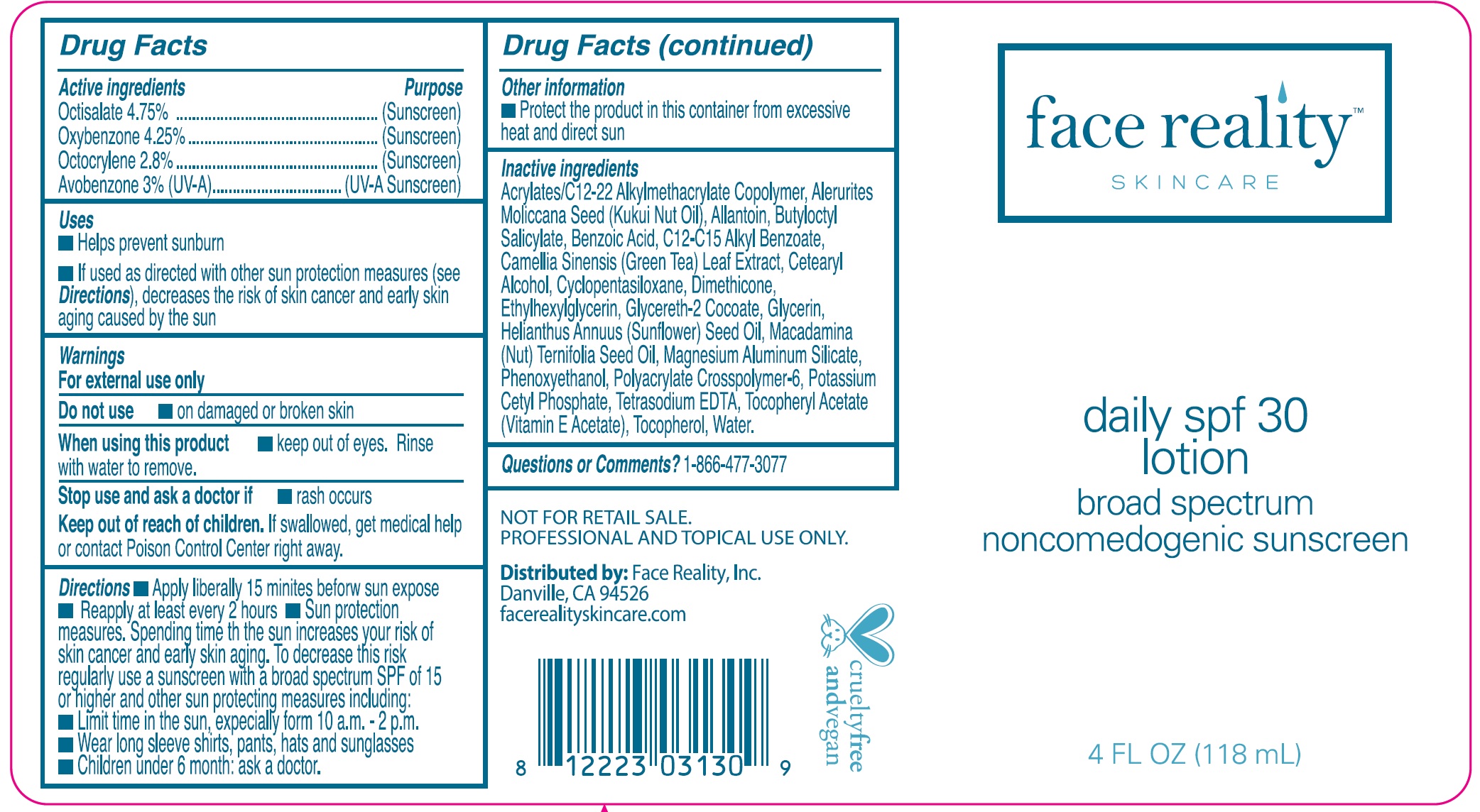
Daily SPF 30 by Face Reality, Inc. Daily SPF 30 Lotion
Daily SPF 30 by
Drug Labeling and Warnings
Daily SPF 30 by is a Otc medication manufactured, distributed, or labeled by Face Reality, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DAILY SPF 30- octisalate, oxybenzone, octocrylene, avobenzone cream
Face Reality, Inc.
----------
Daily SPF 30 Lotion
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see ), decreases the risk of skin cancer and early skin aging caused by the sun Directions
Directions
- Apply liberally 15 minites before sun expose
- Reapply at least every 2 hours
- Sun protection measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protecting measures including:
- Limit time in the sun, expecially form 10 a.m. – 2 p.m.
- Wear long sleeve shirts, pants, hats and sunglasses
- Children under 6 month: ask a doctor.
Inactive ingredients
Acrylate/C 12-22 Alkylmethacrylate Copolymer, Alerurites Moliccana Seed (Kukui Nut Oil), Allantoin, Butyloctyl Salicylate, Benzoic Acid, C12-C15 Alkyl Benzoate, Camellia Sinensis (Green Tea) Leaf Extract, Cetearyl Alcohol, Cyclopentasiloxane, Dimethicone, Ethylhexylglycerin, Glycereth-2 Cocoate, Glycerin, Helianthus Annuus (Sunflower) Seed Oil, Macadamina (Nut) Ternifolia Seed Oil, Magnesium Aluminum Silicate, Phenoxyethanol, Polyacrylate Crosspolymer-6, Potassium Cetyl Phosphate, Tetrasodium EDTA, Tocopheryl Acetate (Vitamin E Acetate), Tocopherol, Water.
| DAILY SPF 30
octisalate, oxybenzone, octocrylene, avobenzone cream |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Face Reality, Inc. (602958071) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.