Ampicillin by Aurobindo Pharma Limited AMPICILLIN capsule
Ampicillin by
Drug Labeling and Warnings
Ampicillin by is a Prescription medication manufactured, distributed, or labeled by Aurobindo Pharma Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
To reduce the development of drug-resistant bacteria and maintain the effectiveness of ampicillin and other antibacterial drugs, ampicillin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
DESCRIPTION
Ampicillin trihydrate is a semisynthetic penicillin derived from the basic penicillin nucleus, 6-aminopenicillanic acid. Ampicillin is designated chemically as (2S, 5R, 6R)-6-[(R)-2-Amino-2-phenylacetamido]-3,3- dimethyl-7-oxo-4-thia-1-azabicyclo [3.2.0] heptane-2-carboxylic acid. Its structural formula is:
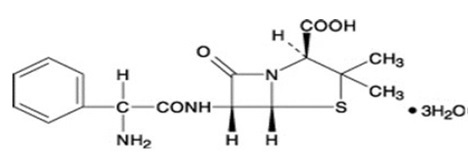
Molecular formula: C16H19N3O4S · 3 H2O
Molecular weight: 403.5
Ampicillin capsules, USP for oral administration provide ampicillin trihydrate equivalent to 250 mg and 500 mg ampicillin USP. Inactive ingredients: ammonium hydroxide, black iron oxide, gelatin, magnesium stearate, potassium hydroxide, propylene glycol, shellac, sodium lauryl sulfate and titanium dioxide.
Meets USP dissolution test 2. -
CLINICAL PHARMACOLOGY
Ampicillin is bactericidal at low concentrations and is clinically effective not only against the gram-positive organisms usually susceptible to penicillin G but also against a variety of gram-negative organisms. It is stable in the presence of gastric acid and is well absorbed from the gastrointestinal tract. It diffuses readily into most body tissues and fluids; however, penetration into the cerebrospinal fluid and brain occurs only with meningeal inflammation. Ampicillin is excreted largely unchanged in the urine; its excretion can be delayed by concurrent administration of probenecid which inhibits the renal tubular secretion of ampicillin. In blood serum, ampicillin is the least bound of all the penicillins; an average of about 20 percent of the drug is bound to plasma proteins as compared to 60 to 90 percent of the other penicillins. The administration of 500 mg dose of ampicillin capsules results in an average peak blood serum level of approximately 3.0 mcg/mL.
Microbiology
Mechanism of Action
Ampicillin is similar to penicillin in its bactericidal action against susceptible bacteria during the stage of active multiplication. It acts through the inhibition of cell wall biosynthesis that leads to the death of the bacteria.
Mechanism of Resistance
Resistance to ampicillin is mediated primarily through enzymes called beta-lactamases that cleave the beta-lactam ring of ampicillin, rendering it inactive.
Antimicrobial Activity
Ampicillin has been shown to be active against most isolates of the following bacteria, both in vitro and in clinical infections, as described in the INDICATIONS AND USAGE section.
Gram-positive Bacteria
Enterococcus spp.
Staphylococcus spp. (non-penicillinase-producing)
Streptococcus pneumoniae
Streptococcus pyogenes
Viridans group streptococci
Gram-negative Bacteria
Escherichia coli
Haemophilus influenzae (non-penicillinase-producing)
Neisseria gonorrhoeae
Neisseria meningitidis
Proteus mirabilis
Salmonella spp.
Shigella spp.
The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following bacteria exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to 0.12 mcg/mL for ampicillin. However the efficacy of ampicillin in treating clinical infections due to these bacteria has not been established in adequate and well-controlled trials.
Gram-positive Bacteria
Bacillus anthracis
Corynebacterium xerosis
Anaerobic Bacteria
Clostridium spp.
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
-
INDICATIONS AND USAGE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of ampicillin capsules and other antibacterial drugs, ampicillin capsules should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. When culture and susceptibility information are available, they should be considered in selecting of modifying antimicrobial therapy, in the absence of such data, local epidemiology and susceptibility patterns contribute to the empiric selection of therapy.
Ampicillin capsules are indicated in the treatment of infections caused by susceptible strains of the designated organisms listed below:
Infections of the genitourinary tract including gonorrhea: E. coli, P. mirabilis, enterococci, Shigella, S. typhosa and other Salmonella, and nonpenicillinase producing N. gonorrhoeae.
Infections of the respiratory tract: Nonpenicillinase- producing H. influenzae and staphylococci, and streptococci including streptococcus pneumoniae.
Infections of the gastrointestinal tract: Shigella, S. typhosa and other Salmonella, E. coli, P. mirabilis, and enterococci.
Meningitis: N. Meningitides.
Bacteriology studies to determine the causative organisms and their susceptibility to ampicillin capsules should be performed. Therapy may be instituted prior to the results of susceptibility testing.
-
CONTRAINDICATIONS
The use of ampicillin is contraindicated in individuals with a history of serious hypersensitivity reactions (e.g., anaphylaxis or Stevens-Johnson syndrome) to ampicillin or to other beta-lactam antibacterial drugs. Ampicillin is also contraindicated in infections caused by penicillinase-producing organisms.
Ampicillin is contraindicated in patients with a previous history of cholestatic jaundice/hepatic dysfunction associated with treatment with ampicillin.
-
WARNINGS
SERIOUS AND OCCASIONALLY FATAL HYPERSENSITIVITY (ANAPHYLACTIC) REACTIONS HAVE BEEN REPORTED IN PATIENTS ON PENICILLIN THERAPY. ALTHOUGH ANAPHYLAXIS IS MORE FREQUENT FOLLOWING PARENTERAL THERAPY, IT HAS OCCURRED IN PATIENTS ON ORAL PENICILLINS. THESE REACTIONS ARE MORE LIKELY TO OCCUR IN INDIVIDUALS WITH A HISTORY OF SENSITIVITY TO MULTIPLE ALLERGENS.
THERE HAVE BEEN REPORTS OF INDIVIDUALS WITH A HISTORY OF PENICILLIN HYPERSENSITIVITY WHO HAVE EXPERIENCED SEVERE REACTIONS WHEN TREATED WITH CEPHALOSPORINS. BEFORE THERAPY WITH ANY PENICILLIN, CAREFUL INQUIRY SHOULD BE MADE CONCERNING PREVIOUS HYPERSENSITIVITY REACTIONS TO PENICILLINS, CEPHALOSPORINS OR OTHER ALLERGENS. IF AN ALLERGIC REACTION OCCURS, APPROPRIATE THERAPY SHOULD BE CONSIDERED.
SERIOUS ANAPHYLACTIC REACTIONS REQUIRE IMMEDIATE EMERGENCY TREATMENT WITH EPINEPHRINE. OXYGEN, INTRAVENOUS STEROIDS, AND AIRWAY MANAGEMENT, INCLUDING INTUBATION, SHOULD ALSO BE ADMINISTERED AS INDICATED.
Severe Cutaneous Adverse Reactions
Ampicillin may cause severe cutaneous adverse reactions (SCARs), such as toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS), drug reaction with eosinophilia and systemic symptoms (DRESS), exfoliative dermatitis, erythema multiforme, and acute generalized exanthematous pustulosis (AGEP). If patients develop a skin rash, they should be monitored closely, and ampicillin discontinued if lesions progress.
Hepatotoxicity
Hepatic dysfunction, including hepatitis and cholestatic jaundice has been associated with the use of ampicillin. Hepatic toxicity is usually reversible; however, deaths have been reported. Hepatic function should be monitored at regular intervals in patients with hepatic impairment.
Clostridioides difficile-Associated Diarrhea
Clostridioides difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including ampicillin, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial drug use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial drug use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
-
PRECAUTIONS
General
A high percentage of patients with mononucleosis who receive ampicillin develop a skin rash. Thus, ampicillin class antibacterial should not be administered to patients with mononucleosis. In patients treated with ampicillin the possibility of superinfections with mycotic or bacterial pathogens should be kept in mind during therapy. If superinfections occur (usually involving Pseudomonas or Candida), the drug should be discontinued and/or appropriate therapy instituted.
Prescribing ampicillin in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Prolonged use of antibiotics may promote the overgrowth of nonsusceptible organisms, including fungi. Should superinfection occur, appropriate measures should be taken.
Patients with gonorrhea who also have syphilis should be given additional appropriate parenteral penicillin treatment.
Treatment with ampicillin does not preclude the need for surgical procedures, particularly in staphylococcal infections.
Information for the Patient
1. The patient should inform the physician of any history of sensitivity to allergens, including previous hypersensitivity reactions to penicillins and cephalosporins (see WARNINGS).
2. Advise patients about the signs and symptoms of serious skin manifestations. Instruct patients to stop taking ampicillin immediately and promptly report the first signs or symptoms of skin rash, mucosal lesions, or any other sign of hypersensitivity [see WARNINGS].
3. Diarrhea is a common problem caused by antibacterials which usually ends when the antibacterial is discontinued. Sometimes after starting treatment with antibacterials, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibacterial. If this occurs, patients should contact their physician as soon as possible.
4. Ampicillin should be taken with a full glass (8 oz) of water, one-half hour before or two hours after meals.
5. Diabetic patients should consult with the physician before changing diet or dosage of diabetes medication (see PRECAUTIONS–Drug/Laboratory Test Interactions).
6. Patients should be counseled that antibacterial drugs including ampicillin should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When ampicillin is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may: (1) decrease the effectiveness of the immediate treatment, and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by ampicillin or other antibacterial drugs in the future.
Laboratory Tests
In prolonged therapy, and particularly with high dosage regimens, periodic evaluation of the renal, hepatic, and hematopoietic systems is recommended.
In streptococcal infections, therapy, must be sufficient to eliminate the organism (10 days minimum); otherwise the sequelae of streptococcal disease may occur. Cultures should be taken following completion of treatment to determine whether streptococci have been eradicated.
Cases of gonococcal infection with a suspected lesion of syphilis should have darkfield examinations ruling out syphilis before receiving ampicillin. Patients who do not have suspected lesions of syphilis and are treated with ampicillin should have a follow-up serologic test for syphilis each month for four months to detect syphilis that may have been masked from treatment for gonorrhea.
Drug Interactions
When administered concurrently, the following drugs may interact with ampicillin.
Allopurinol: Substantially increased incidence of skin rashes in patients receiving both drugs as compared to patients receiving ampicillin alone. It is not known whether this potentiation of ampicillin rashes is due to allopurinol or the hyperuricemia present in these patients.
Bacteriostatic Antibiotics: Chloramphenicol, erythromycins, sulfonamides, or tetracyclines may interfere with the bactericidal effect of penicillins. This has been demonstrated in vitro; however, the clinical significance of this interaction is not well-documented.
Oral Contraceptives: May be less effective and increased breakthrough bleeding may occur.
Probenecid: May decrease renal tubular secretion of ampicillin resulting in increased blood levels and/or ampicillin toxicity.
Drug/Laboratory Test Interactions
After treatment with ampicillin, a false-positive reaction for glucose in the urine may occur with copper sulfate tests (Benedict’s solution, Fehling’s solution, or Clinitest® tablets) but not with enzyme based tests such as Clinistix® and Tes-Tape®. Following administration of ampicillin to pregnant women, a transient decrease in plasma concentration of total conjugated estriol, estriol-glucuronide, conjugated estrone and estradiol has been noted.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenesis, mutagenesis, or impairment of fertility in males or females.
Pregnancy
Teratogenic Effects
Reproduction studies in animals have revealed no evidence of impaired fertility or harm to the fetus due to penicillin. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, penicillin should be used during pregnancy only if clearly needed. (See PRECAUTIONS –Drug/Laboratory Test Interactions.)
Labor and Delivery
Oral ampicillin-class antibiotics are poorly absorbed during labor. Studies in guinea pigs showed that intravenous administration of ampicillin slightly decreased the uterine tone and frequency of contractions, but moderately increased the height and duration of contractions. However, it is not known whether use of these drugs in humans during labor or delivery has immediate or delayed adverse effects on the fetus, prolongs the duration of labor, or increases the likelihood that forceps delivery or other obstetrical intervention or resuscitation of the newborn will be necessary.
Nursing Mothers
Ampicillin-class antibiotics are excreted in milk. Ampicillin used by nursing mothers may lead to sensitization of infants; therefore, caution should be exercised when ampicillin is administered to a nursing woman.
Pediatric Use
Penicillins are excreted primarily unchanged by the kidney; therefore, the incompletely developed renal function in neonates and young infants will delay the excretion of penicillin. Administration to neonates and young infants should be limited to the lowest dosage compatible with an effective therapeutic regimen (see DOSAGE AND ADMINISTRATION).
-
ADVERSE REACTIONS
As with other penicillins, it may be expected that untoward reactions will be essentially limited to sensitivity phenomena. They are more likely to occur in individuals who have previously demonstrated hypersensitivity to penicillin and in those with a history of allergy, asthma, hay fever, or urticaria.
The following adverse reactions have been reported as associated with the use of ampicillin:
Infections and Infestations: Clostridioides difficile-associated diarrhea (see WARNINGS section).
Gastrointestinal: glossitis, stomatitis, nausea, vomiting, enterocolitis, pseudomembranous colitis, and diarrhea. These reactions are usually associated with oral dosage forms of the drugs.
Hypersensitivity Reactions: Severe cutaneous adverse reactions, including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), angioedema, and acute generalized exanthematous pustulosis (AGEP) have been reported in beta-lactam antibiotics (see WARNINGS). An erythematous, mildly pruritic, maculopapular skin rash has been reported fairly frequently. The rash, which usually does not develop within the first week of therapy, may cover the entire body including the soles, palms, and oral mucosa. The eruption usually disappears in three to seven days. Other hypersensitivity reactions that have been reported are: skin rash, pruritus, urticaria, erythema multiforme, and an occasional case of exfoliative dermatitis. Linear IgA bullous dermatosis has been reported. Anaphylaxis is the most serious reaction experienced and has usually been associated with the parenteral dosage form.
NOTE: Urticaria, other skin rashes, and serum sickness-like reactions may be controlled by antihistamines, and, if necessary, systemic corticosteroids. Whenever such reactions occur, ampicillin should be discontinued unless, in the opinion of the physician, the condition being treated is life-threatening, and amenable only to ampicillin therapy. Serious anaphylactic reactions require emergency measures (see WARNINGS).
Liver: Moderate elevation in serum glutamic oxaloacetic transaminase (SGOT) has been noted, but the significance of this finding is unknown.
Hemic and Lymphatic Systems: Anemia, thrombocytopenia, thrombocytopenic purpura, eosinophilia, leukopenia, and agranulocytosis have been reported during therapy with penicillins. These reactions are usually reversible on discontinuation of therapy and are believed to be hypersensitivity phenomena.
Other adverse reactions that have been reported with the use of ampicillin are laryngeal stridor and high fever. An occasional patient may complain of sore mouth or tongue as with any oral penicillin preparation.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
Adults and pediatric patients weighing over 20 kg:
For genitourinary or gastrointestinal tract infections other than gonorrhea in men and women, the usual dose is 500 mg qid in equally spaced doses; severe or chronic infections may require larger doses. For the treatment of gonorrhea in both men and women, a single oral dose of 3.5 grams of ampicillin administered simultaneously with 1 gram of probenecid is recommended. Physicians are cautioned to use no less than the above recommended dosage for the treatment of gonorrhea. Follow-up cultures should be obtained from the original site(s) of infection 7 to 14 days after therapy. In women, it is also desirable to obtain culture test-of-cure from both the endocervical and anal canals. Prolonged intensive therapy is needed for complications such as prostatitis and epididymitis.
For respiratory tract infections, the usual dose is 250 mg qid in equally spaced doses.
Pediatric patients weighing 20 kg or less:
For genitourinary or gastrointestinal tract infections, the usual dose is 100 mg/kg/day total, qid in equally divided and spaced doses. For respiratory tract infections, the usual dose is 50 mg/kg/day total, in equally divided and spaced doses three to four times daily. Doses for children should not exceed doses recommended for adults.
All Patients, Irrespective of Age and Weight:
Larger doses may be required for severe or chronic infections. Although ampicillin is resistant to degradation by gastric acid, it should be administered at least one-half hour before or two hours after meals for maximal absorption. Except for the single dose regimen for gonorrhea referred to above, therapy should be continued for a minimum of 48 to 72 hours after the patient becomes asymptomatic or evidence of bacterial eradication has been obtained. In infections caused by hemolytic strains of streptococci, a minimum of 10 days’ treatment is recommended to guard against the risk of rheumatic fever or glomerulonephritis (see PRECAUTIONS–Laboratory Tests). In the treatment of chronic urinary or gastrointestinal infections, frequent bacteriologic and clinical appraisal is necessary during therapy and may be necessary for several months afterwards. Stubborn infections may require treatment for several weeks. Smaller doses than those indicated above should not be used.
-
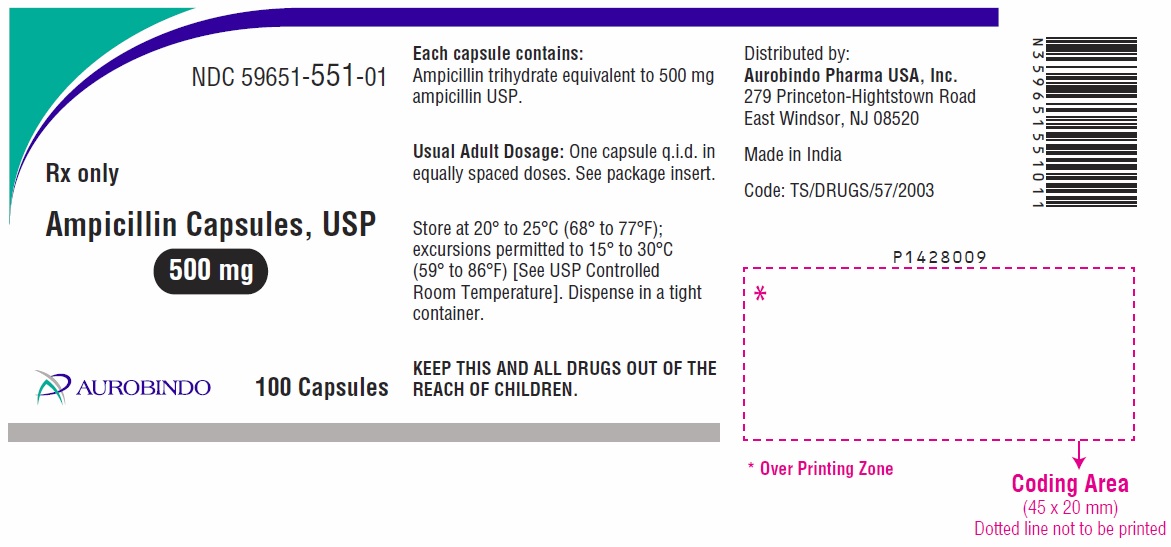
HOW SUPPLIED
Ampicillin capsules, USP: Each capsule, for oral administration, contains ampicillin trihydrate equivalent to 250 mg or 500 mg ampicillin USP, and are supplied as:
250 mg: white opaque colored cap and body, hard gelatin capsule filled with white to off-white granular powder, imprinted with “AMP” on cap and “250” on body, with black ink.
Bottles of 100 NDC: 59651-550-01
Bottles of 500 NDC: 59651-550-05500 mg: white opaque colored cap and body, hard gelatin capsule filled with white to off-white granular powder, imprinted with “AMP” on cap and “500” on body, with black ink.
Bottles of 100 NDC: 59651-551-01
Bottles of 500 NDC: 59651-551-05
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
Dispense in a tight container.
All brands listed are the trademarks of their respective owners and are not trademarks of Aurobindo Pharma Limited.
Distributed by:
Aurobindo Pharma USA, Inc.
279 Princeton-Hightstown Road
East Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited
Hyderabad-500 032, India
Revised: 08/2025 - PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 250 mg (100 Capsules Bottle)
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 500 mg (100 Capsules Bottle)
-
INGREDIENTS AND APPEARANCE
AMPICILLIN
ampicillin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59651-550 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMPICILLIN TRIHYDRATE (UNII: HXQ6A1N7R6) (AMPICILLIN - UNII:7C782967RD) AMPICILLIN 250 mg Inactive Ingredients Ingredient Name Strength AMMONIA (UNII: 5138Q19F1X) FERROSOFERRIC OXIDE (UNII: XM0M87F357) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) MAGNESIUM STEARATE (UNII: 70097M6I30) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (Opaque) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code AMP;250 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59651-550-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/31/2023 2 NDC: 59651-550-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 10/31/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA216554 10/31/2023 AMPICILLIN
ampicillin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59651-551 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMPICILLIN TRIHYDRATE (UNII: HXQ6A1N7R6) (AMPICILLIN - UNII:7C782967RD) AMPICILLIN 500 mg Inactive Ingredients Ingredient Name Strength AMMONIA (UNII: 5138Q19F1X) FERROSOFERRIC OXIDE (UNII: XM0M87F357) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) MAGNESIUM STEARATE (UNII: 70097M6I30) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (Opaque) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code AMP;500 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59651-551-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/31/2023 2 NDC: 59651-551-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 10/31/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA216554 10/31/2023 Labeler - Aurobindo Pharma Limited (650082092) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 918917683 ANALYSIS(59651-550, 59651-551) , MANUFACTURE(59651-550, 59651-551)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.